| Pipeline Report - HER2 inhibitors | |
| Drug | Common Drug Name | Database | Synonyms | Originator | Highest Phase | Mechanism Of Action | Structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | AC-480 | BMS 599626 |
|
EGFR kinase inhibitors, Ambit | Bristol-Myers Squibb | Phase I | HER4 (erbB4) Inhibitors EGFR (HER1 erbB1) Inhibitors HER2 (erbB2) Inhibitors |
 |
||||||||||||
| 1.4 PharmProj | 1.4 PharmProj | 1.1 Adis RDI | 1.1 Adis RDI | 1.3 TR Integ | 1.2 TPharma | |||||||||||||||
| 2. | canertinib dihydrochloride | Canertinib dihydrochloride |
|
CI-1033 PD-0183805 PD-183805 |
University of Auckland | Phase II | Anticancer Apoptosis stimulator Erbb2 tyrosine kinase receptor inhibitor Erbb3 tyrosine kinase receptor inhibitor Erbb4 tyrosine kinase receptor inhibitor AKT protein kinase inhibitor Angiogenesis inhibitor Epidermal growth factor antagonist |
 |
||||||||||||
| 2.3 PharmProj | 2.3 PharmProj | 2.1 TPharma | 2.2 TR Integ | 2.1 TPharma | 2.1 TPharma | |||||||||||||||
| 3. | D-69491 | D-69491 |
|
SU-11464 | SUGEN Inc | Preclinical | Anticancer Erbb2 tyrosine kinase receptor inhibitor Epidermal growth factor antagonist |
 |
||||||||||||
| 3.3 PharmProj | 3.3 PharmProj | 3.1 TPharma | 3.2 TR Integ | 3.1 TPharma | 3.1 TPharma | |||||||||||||||
| 4. | DXL-702 | DXL-702 |
|
InNexus Biotechnology | Preclinical | biotechnology protein monoclonal antibody |
||||||||||||||
| 4.3 PharmProj | 4.1 Adis RDI | 4.1 Adis RDI | 4.4 IMS RDF | |||||||||||||||||
| 5. | E-75 | E-75 |
|
APT-101 NeuVax |
Henry M. Jackson Foundation University of Washington |
Phase III Clinical Trial | vaccine peptide |
 |
||||||||||||
| 5.2 PharmProj | 5.2 PharmProj | 5.1 TR Integ | 5.2 PharmProj | 5.3 IMS RDF | 5.1 TR Integ | |||||||||||||||
| 6. | erbB2 tyrosine kinase receptor inhibitor (cancer), AstraZeneca | erbB2 tyrosine kinase receptor inhibitor (cancer), AstraZeneca |
|
erbB2 kinase inhibitors (cancer), AstraZeneca IDDBCP188446 IDDBCP199515 |
AstraZeneca plc | Discovery | Anticancer Erbb2 tyrosine kinase receptor inhibitor Epidermal growth factor antagonist |
 |
||||||||||||
| 6.1 TPharma | 6.1 TPharma | 6.1 TPharma | 6.1 TPharma | 6.1 TPharma | 6.1 TPharma | |||||||||||||||
| 7. | PX-104.1 | HER-2 Protein AutoVac |
|
anti-HER-2 protein, Pharmexa HER-2 protein AutoVac,Pharmacc ME-104 |
Pharmexa A/S | Phase II Clinical Trial | Therapeutic vaccine Anticancer Erbb2 tyrosine kinase receptor inhibitor Epidermal growth factor antagonist |
|||||||||||||
| 7.3 PharmProj | 7.3 PharmProj | 7.1 TPharma | 7.3 PharmProj | 7.1 TPharma | ||||||||||||||||
| 8. | HKI-357 | HKI-357 |
|
CPD-820 WAY-177820 compound 820, Wyeth-Ayerst EGF-R kinase inhibitors (oral, cancer), Wyeth-Ayerst erb-B2 inhibitors (oral, cancer), Wyeth-Ayerst HER-2 inhibitors (oral, cancer), Wyeth HER-2 inhibitors (oral, cancer), Wyeth-Ayerst tyrosine kinase inhibitors (oral, cancer), Wyeth-Ayerst |
Wyeth | Discovery | Anticancer Erbb2 tyrosine kinase receptor inhibitor Epidermal growth factor antagonist |
 |
||||||||||||
| 8.1 TPharma | 8.1 TPharma | 8.1 TPharma | 8.1 TPharma | 8.1 TPharma | 8.1 TPharma | |||||||||||||||
| 9. | IDM-1 | IDM-1 |
|
Osidem Osidem-2 MAK + anti-HER2/neu bispecific antibody MAK + MDX-210 |
Medarex | Phase III | Anti-HER2/neu/ErbB2 Anti-FCgammaRI (FCGR1 CD64) Inhibitors of Signal Transduction Pathways |
|||||||||||||
| 9.2 TPharma | 9.2 TPharma | 9.1 Adis RDI | 9.3 TR Integ | 9.4 TR Integ | ||||||||||||||||
| 10. | RB-200h | RB-200h |
|
Dimercept Hermodulins Herstatin |
Receptor BioLogix Inc | Preclinical | Anticancer Epidermal growth factor receptor modulator Erbb2 tyrosine kinase receptor inhibitor Erbb3 tyrosine kinase receptor inhibitor Erbb4 tyrosine kinase receptor inhibitor Oncogene inhibitor |
|||||||||||||
| 10.2 PharmProj | 10.2 PharmProj | 10.1 TPharma | 10.2 PharmProj | 10.1 TPharma | ||||||||||||||||
| 11. | Zemab | Zemab |
|
anticancer antibody, G2M | Novartis | Phase II Clinical Trial | biotechnology monoclonal antibody monoclonal antibody fragment immunotoxin fusion toxin |
|||||||||||||
| 11.3 PharmProj | 11.3 PharmProj | 11.1 Adis RDI | 11.3 PharmProj | 11.4 IMS RDF | ||||||||||||||||
| Publisher Version | Back to chart | Next record |
-
BMS 599626
(AC 480, AC480, BMS-599626)
- Drug Development Phase
Indication Phase Country Solid tumours (Metastatic disease) Phase I USA - Properties
- Mechanism Of Action: Epidermal growth factor inhibitors; HER2 inhibitors
Pharmacodynamics: Efficacy in murine HER-overexpressing antitumour model; inhibits HER1 and HER2 in breast cancer cell line; dose-dependently inhibits HER1, HER2 and HER3 phosphorylation across a panel of HER1 and/or HER2 expressing tumour cell lines
Route of Administration: PO - Commercial Introduction
- Bristol-Myers Squibb and Ambit Biosciences Corporation are co-developing BMS 599626 for the treatment of cancer. The orally administered, pyrrolotriazine compound is a dual inhibitor of both epidermal growth factor receptor (EGFR/ErbB1/HER1) and HER2 (ErbB2/neu) protein tyrosine kinases. EGFR and HER2 have been found to be frequently overexpressed in a variety of tumour types. BMS 599626 is in clinical development for solid tumours in the US.
BMS 599626 emerged from a collaborative research programme between Bristol-Myers Squibb and Ambit Biosciences which was originally signed in January 2005; the kinase inhibitor was identified using Ambit's proprietary kinase profiling technology (KinomeScan[trademark]). KinomeScan[trademark] is an innovative, high-throughput method for screening small molecule libraries against a large number of human kinases. - Review
- Pharmacokinetics
- In a phase I trial in patients receiving a single dose of BMS 599626 21 days on and 7 days off, Tmax ranged from 1-8h. Following a single dose, geomean Cmax = 162 ng/mL, mean AUC0-[infinity] = 2920 ng/mL h. Day 8 and day 21 revealed similar Cmax and AUC0-[infinity] values suggesting no significant accumulation in exposure over time. Terminal half-life was approximately 20h. Exposure increase was linear from 100mg to 200mg dose levels<REF href='adis://adnm/800975801' /><REF href='adis://adnm/800975788' />.
- Pharmacodynamics
- In BT474 breast cancer cells, inhibited both HER1 and HER2 with IC50 values of 0.022 and 0.032 ?mol/L, respectively. In N87 cells, BMS 599626 inhibited HER2 autophosphorylation, activation of MAPK and Akt signalling. In HER-overexpressing murine tumour models, BMS 599626 had antitumour efficacy from 60-240 mg/kg and was more effective than gemcitabine<REF href='adis://adnm/801005603' />.
BMS 599626 showed dose-dependent inhibition of HER1, HER2 and HER3 phosphorylation across a panel of HER1 and/or HER2 expressing tumour cell lines, which included BT474, SkBr3, A431, and MDA-MB-468 (IC50 values varied with cell line). This led to BMS 599626-induced inhibition of HER1 and HER2 overexpressing cancer cell lines. As with receptor phosphorylation, BMS 599626 displayed dose-dependent attenuation of the AKT and Ras/MAPK pathways in a cell type-dependent manner via inhibition of downstream signalling triggered by HER activation<REF href='adis://adnm/801109474' />. - Drug Development History
Date Event 16 Apr 2008 Pharmacodynamics data from preclinical trials presented at the 99th Annual Meeting of the American Association for Cancer Research (AACR-2008) 16 Nov 2007 BMS 599626 is still in phase I trials for metastatic solid tumours in USA 18 May 2005 Data presented at the 41st Annual Meeting of the American Society of Clinical Oncology (ASCO-2005) have been added to the adverse events, pharmacokinetics and Cancer therapeutic trials sections (, ) 29 Mar 2005 Data presented at the 229th American Chemical Society National Meeting (229th-ACS-2005) have been added to the Cancer pharmacodynamics section 29 Mar 2005 Phase-I clinical trials in Solid tumours in USA (PO) 13 Jan 2005 New profile 13 Jan 2005 Preclinical trials in Cancer in USA (PO)
Last Update: 2008-05-08
Adis R&D Insight accesion number: 800022263
Indications: Solid tumours (Metastatic disease)
Therapeutic Class (WHO): Antineoplastic Agents (L01)
Therapeutic Class (EphMRA): Antineoplastics (L1)
Originator: Bristol-Myers Squibb
Licensee: Ambit Biosciences Corporation
Highest Phase: Phase I
© 2008 Adis Data Information BV
| Publisher Version | Back to chart | Previous record | Next record |
-
AC-480
- Investigational Drugs Database 52197
- Companies
- Bristol-Myers Squibb Co: Originator developing and marketing own product
Ambit Biosciences Corp: Licensee for development and marketing - Development Status
Company Country Status Indication Date Confidence Ambit Biosciences Corp US Discovery Cancer 2005-12-12 Bristol-Myers Squibb Co US Discontinued Cancer 2005-12-12
Status: Discovery- Summary
- Ambit Biosciences, presumed to be under license from Bristol-Myers Squibb, is investigating AC-480 (formerly BMS-599626), an orally active inhibitor of multiple HER tyrosine kinases, for the potential treatment of cancer [ 639937], [ 895412]. In April 2008, preclinical data were presented [ 895412].
- Latest Change
- 2008-05-02: 1 Reference Added [902209]
(BMS-599626, HER kinase inhibitor, Bristol-Myers Squibb, pan HER kinase inhibitor, BMS, pan-HER inhibitor (cancer), Ambit)
CAS Registry No.: 911701-24-1

Update Date: 2008-05-02
http://www.thomson-pharma.com/report/drug?dr=52197&drname=AC-480
Indication: Cancer
Action: Anticancer; EGFR family tyrosine kinase receptor inhibitor; Erbb2 tyrosine kinase receptor inhibitor; Erbb4 tyrosine kinase receptor inhibitor; Epidermal growth factor antagonist
Technology: Oral formulation
Copyright Thomson Scientific
| Publisher Version | Back to chart | Previous record | Next record |
-
BMS-599626
- Development Status
- Development Status - Launched, Registered or Under Active Development
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Phase I Bristol-Myers Squibb Cancer, solid tumor In patients with advanced solid malignancies that express Her2. - Therapeutic Class
- Oncolytic Drugs
- Condition
- Cancer, solid tumor
- Action
- EGFR (HER1; erbB1) Inhibitors; HER2 (erbB2) Inhibitors; Inhibitors of Signal Transduction Pathways
- Summary
- BMS-599626 is a small molecule inhibitor of the human epidermal growth factor receptor (HER) kinase family in early clinical trials at Bristol-Myers Squibb for the treatment of metastatic solid tumors. The drug candidate targets both the HER1 and the HER2 receptors, which are frequently co-expressed in a range of tumor types and possess the ability to form heterodimers. In previous studies, BMS-599626 demonstrated efficacy in HER2-expressing tumor models that are sensitive or resistant to trastuzumab, and showed good oral bioavailability in animal models.
- Related Patents
- WO 2004054514
Prous Accession Number: 393950

Registry Number: 714971-09-2 714971-14-9 (undefined isomer)
Molecular Formula: C27H28ClFN8O3
Molecular Weight: 567.0144
Originator: Bristol-Myers Squibb
Highest Phase: Phase I
Copyright Prous Science
| Back to chart | Previous record | Next record |
-
AC-480
(EGFR kinase inhibitors, Ambit)
- Company Status
Company Status Ambit Biosciences Preclinical - Summary
- AC-480 is a small-molecule pan-EGFR kinase inhibitor, under development by Ambit Biosciences for the treatment of solid tumours such as lung or breast cancer. It is active against EGFR, HER2, HER3 and HER4 (BIO 2005 (Philadelphia); JP Morgan 26th Ann Healthcare Conf (San Francisco), 2008).
Clinical
Phase II
Phase I/II trials in solid tumours to establish safety, pharmacokinetics and tolerability are expected in the 2nd qtr of 2008.
Preclinical
In preclinical studies, it demonstrated efficacy in breast, lung, pancreatic and head and neck cancers (JP Morgan 26th Ann Healthcare Conf (San Francisco), 2008).
Licensing
Ambit was previously seeking a partner for clinical development and commercialization (12th BioPartner Eur (London), 2004). Updated by JM on 14/1/2008. - Therapeutic Activity
- Anticancer, other (K6Z)
- Pharmacological Activity
- ErbB-1 tyrosine kinase inhibitor (KI-TYE1-AN)
ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
ErbB-4 tyrosine kinase inhibitor (KI-TYE4-AN)
Epidermal growth factor receptor 3 inhibitor (GF-EPR3-AN) - Indications
- Unspecified
- Target Names
- epidermal growth factor receptor (erythroblastic leukaemia viral (v-erb-b) oncogene homologue, avian)
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 3 (avian)
v-erb-a erythroblastic leukaemia viral oncogene homologue 4 (avian) - Therapy Status
Therapy Pharmacology Status K6Z KI-TYE1-AN KI-TYE2-AN KI-TYE4-, GF-EPR3-AN Preclinical - Indication Status
Indication Status Unspecified Preclinical - Regional Status
- USA - Preclinical
- Ratings
- Novelty: 4 - Low Novelty
Market Size: 3 - US$ 2001-5000 million
Development Speed: 1 - Development not started
Total: 8 - Update History
Date Detail 2008-01-14 Compounds Identified (EGFR kinase inhibitors, Ambit) 2008-01-14 Development Continuing 2006-10-16 No Development Reported 2005-06-21 Target Identified (epidermal growth factor receptor (1956)) 2005-06-21 Pharmacology Identified (ErbB-1 inhibitor (KI-TY-TIE-)) 2004-10-18 New Product in Pharmaprojects 2004-10-01 Licensing Opportunities (Worldwide)
PJB Pharmaprojects Accession Number: 39178
Last Update: 2008-01-14
Originator: Ambit Biosciences (USA)
World Status: Preclinical
Origin of Material: Chemical, synthetic (CH-SY)
Route of Administration: Unknown (UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record | Next record |
-
BMS-599626
(HER1/2 inhibitors, BMS)
- Company Status
Company Status Bristol-Myers Squibb No Development Reported - Summary
- BMS-599626 is a small-molecule dual kinase inhibitor which targets the HER1 and HER2 receptors, which was under development by Bristol-Myers Squibb for the treatment of breast cancer (229th ACS (San Diego), 2005, MEDI 21).
Clinical
Phase I
Phase I trials were ongoing. It had a t1/2 of 6-7hr, suggesting suitability for once-daily dosing.
Preclinical
In monkeys and dogs, it had po bioavailability of 31 and 49%, respectively. In HER1- or HER2-overexpressing models, BMS-599626 60-240mg/kg po once-daily x14 days showed dose-dependent efficacy. Doses of >120mg/kg resulted in tumour stasis during treatment, and tumour regrowth occurred on cessation of treatment. In a GEO cell line, BMS-599626 60-180mg/kg showed better efficacy cf gefitinib (qv). In HER2 models N87 and KPL4, BMS-599626 60 and 180mg/kg showed dose-dependent efficacy. Efficacy with 180mg/kg was similar to that seen with trastuzumab (qv) 20mg/kg. In N87 cells, BMS-599626 inhibited HER2 phosphorylation and activation of MAP kinase and AKT signalling. Against HER1 and HER2, it had IC50s of 0.022 and 0.032µM, respectively. The IC50 for CYP inhibition was >10µM (229th ACS (San Diego), 2005, MEDI 21). It inhibits HER1 and HER2 via distinct mechanisms and inhibited the proliferation of HER1- and 2-dependent tumour cell lines with IC50s of 0.24-1µM. It also inhibited the formation of HER1/2 heterodimers and downstream signalling (Clin Cancer Res, 2006, 12, 6186, PMID:17062696). SAR studies of a series of nitro-1,4-benzo[1,3,2]dithiazole 3,3-dioxides as small-molecule inhibitors of HER2 kinase were presented at 227th ACS. However, poor metabolic stability, lack of SAR and availability of more promising leads precluded further development of this series (227th ACS (Anaheim), 2004, MEDI 44). Development of dual HER1/HER2 kinase inhibitors was reported at 226th ACS (New York), 2003, MEDI 13. Further pyrrolo[2,1-f][1,2,4] triazine derivatives are reported in Bioorg Med Chem Lett, 16 Aug 2005, PMID:16111887. Updated by IL on 5/1/2007. - Therapeutic Activity
- Anticancer, other (K6Z)
- Pharmacological Activity
- ErbB-1 tyrosine kinase inhibitor (KI-TYE1-AN)
ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN) - Indications
- Cancer, breast
- Target Names
- epidermal growth factor receptor (erythroblastic leukaemia viral (v-erb-b) oncogene homologue, avian)
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian) - Therapy Status
Therapy Pharmacology Status K6Z KI-TYE1-AN KI-TYE2-AN No Development Reported - Indication Status
Indication Status Cancer, breast No Development Reported - Regional Status
- USA - Phase I Clinical Trial
- Ratings
- Novelty: 0 - Not available
Market Size: 3 - US$ 2001-5000 million
Development Speed: 0 - Not available
Total: 0 - Update History
Date Detail 2007-01-22 No Development Reported 2005-03-17 New Chemical Structure (New) 2005-03-13 Compounds Identified (HER-1/2 inhibitors, BMS) 2005-03-13 Change in Status (Phase I Clinical Trial) 2003-09-11 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 36640
Last Update: 2007-01-22
Originator: Bristol-Myers Squibb (USA)
World Status: No Development Reported
Origin of Material: Chemical, synthetic (CH-SY)
Route of Administration: Unknown (UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record |
-
Pan-HER kinase inhibitor, Bristol-Myers Squibb
(BMS 599626)
- IMS R&D Focus Accession Number: 2023713
- Latest Information
- 21 March 2005: Preclinical data from studies involving Bristol-Myers Squibb's BMS 599626, an orally active dual inhibitor of HER1 and HER2 protein tyrosine kinases, were presented at the 229th American Chemical Society national meeting, 13-17 March 2005, San Diego, USA. This compound inhibited HER1 and HER2 at IC50 values of 0.022 mcM and 0.032 mcM, respectively. The agent showed antitumor efficacy in both HER1 and HER2-dependent human tumor xenograft models; no significant weight loss was observed and doses were well tolerated. BMS 599626 had an oral bioavailability of 49% and 31% in dogs and monkeys, respectively, and pharmacokinetic data revealed that the agent had a half-life of 6-7 h, making it suitable for once-daily or twice-daily dosing. ....BMS 599626 is undergoing phase I evaluation in the USA.23 May 2005: Chemical data added.
- Commercial Summary
- Bristol-Myers Squibb is developing BMS 599626, an orally active dual inhibitor of HER1 and HER2 protein tyrosine kinases, as a potential anti tumor agent. The agent is undergoing phase I evaluation in the USA (Bristol-Myers Squibb, NOV 2004).
Preclinical data
In vitro, BMS 599626 had IC50 values of 0.022 mcM and 0.032 mcM against HER1 and HER2, respectively. The agent showed antitumor efficacy in both HER1 and HER2-dependent human tumor xenograft models; no significant weight loss was observed and doses were well tolerated. BMS 599626 had an oral bioavailability of 49% and 31% in dogs and monkeys, respectively, and pharmacokinetic data revealed that the agent had a half-life of 6-7 h (229th ACS, Abs MEDI 0021, MAR 2005). - Indications
- cancer
- Therapeutic Class
- All Other Antineoplastics (L1X9)
- Mechanism of Action
- EGF receptor inhibitor
- Indication Status
Country Indication Phase USA cancer Phase I - Franchise
Company Parent Relation Region Bristol Myers Squibb Bristol Myers Squibb developer - Update History
Date Detail NOV 2004 Phase I, USA.
Publication Date: 2007-01-01
Originator: Bristol Myers Squibb (USA)
Highest Phase: Phase I
Copyright IMS World Publications
| Publisher Version | Back to chart | Next record |
-
canertinib
- Investigational Drugs Database 17772
- Companies
- Pfizer Inc: Licensee for development and marketing
University of Auckland: Originator developing and marketing own product
Parke-Davis & Co (Pfizer Inc): Licensee for development and marketing - Development Status
Company Country Status Indication Date Confidence Pfizer Inc US No Development Reported Cancer 2007-08-21 University of Auckland New Zealand Discontinued Cancer 1998-12-31
Status: No Development Reported- Summary
- As no development has been reported for some time, this program is assumed to be discontinued.
- Latest Change
- 2007-08-21: one or more development status entries have been updated
(canertinib dihydrochloride, CI-1033, SN-26606, PD-0183805, PD-183805)
CAS Registry No.: 267243-28-7 289499-45-2
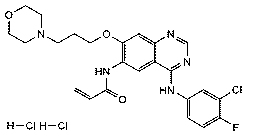
Update Date: 2007-08-21
http://www.thomson-pharma.com/report/drug?dr=17772&drname=canertinib
Indication: Cancer
Action: Anticancer; Apoptosis stimulator; Erbb2 tyrosine kinase receptor inhibitor; Erbb3 tyrosine kinase receptor inhibitor; Erbb4 tyrosine kinase receptor inhibitor; AKT protein kinase inhibitor; Angiogenesis inhibitor; Epidermal growth factor antagonist
Technology: Oral formulation
Copyright Thomson Scientific
| Publisher Version | Back to chart | Previous record | Next record |
-
Canertinib dihydrochloride (free base)
- Development Status
- Development Status - No Development Reported
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Phase II Pfizer Cancer, breast metastatic Phase II Pfizer Cancer, lung (non-small cell) Alone or in combination with paclitaxel and carboplatin - Therapeutic Class
- Non-Small Cell Lung Cancer Therapy; Breast Cancer Therapy
- Condition
- Cancer, breast metastatic; Cancer, lung (non-small cell)
- Action
- HER4 (erbB4) Inhibitors; EGFR (HER1; erbB1) Inhibitors; HER2 (erbB2) Inhibitors; Inhibitors of Signal Transduction Pathways
- Summary
- Canertinib dihydrochloride is a potent and selective, irreversible, small-molecule tyrosine kinase inhibitor which has completed early clinical trials in combination with paclitaxel and carboplatin for the treatment of non-small cell lung cancer (NSCLC). Canertinib blocks signal transduction through all four members of the ErbB (or epidermal growth factor [EGF]) family. The compound had been in phase II trials for the treatment of metastatic breast cancer, however, no recent development at the company has been reported.
- Related Patents
- EP 1230919; US 2004158065; WO 2000031048; WO 2001032155; WO 2001070255; WO 2002000630; WO 2003103676; WO 2004014386; WO 2005003325; WO 2006047716
(CI-1033; PD-183805 (free base))
Prous Accession Number: 274534

Registry Number: 289499-45-2 267243-28-7 (free base) 338796-35-3 (deleted CAS)
Molecular Formula: C24H27Cl3FN5O3
Molecular Weight: 558.8596
Originator: Pfizer
Highest Phase: Phase II
Copyright Prous Science
| Back to chart | Previous record |
-
canertinib dihydrochloride
(CI-1033, PD-0183805, PD-183805)
- Company Status
Company Status Pfizer Discontinued - Summary
- Parke-Davis (Pfizer) has discontinued development of canertinib dihydrochloride (CI-1033), a pan-erbB receptor antagonist for the treatment of nsclc, due to skin toxicity in clinical trials (91st AACR (San Francisco), 2000, Abs 1533; 97th AACR (Washington, DC), 2006, Abs 1306).
Clinical
Phase II
It was previously in Phase II trials (Company presentation, Pfizer, 18 Dec 2001).
Phase I
In an open-label, dose-escalating Phase I trial in 53 patients with advanced non-haematologic malignancies, including lung, colorectal, head and neck, thyroid and breast cancers, mesothelioma, sarcoma and melanoma, canertinib dihydrochloride 10-500mg iv x3days/wk was safe and well tolerated. After 6.8wk of treatment, 53% of patients had SD. DLT's included hypersensitivity and diarrhoea (16th EORTC-NCI-AACR Symp Molec Targ Cancer Ther (Geneva), 2004, Abs 281). In a dose-finding study in 32 previously-treated patients with advanced non-haematologic malignancies, including pancreatic, head and neck, skin, breast and colorectal cancers and nsclc, canertinib dihydrochloride 300-560mg/day po x14 days followed by a 7-day drug-free period produced 10SDs lasting >12wk. Common side-effects were rash, stomatitis and nausea, with 6 cases of Grade 3 diarrhoea (2 of which required treatment discontinuation). The MTD was 450mg and the plasma elimination t1/2 was 4hr (39th ASCO (Chicago), 2003, Abs 974).
Preclinical
In vitro in the human bile duct carcinoma cell line, HuCCT-1, canertinib dihydrochloride dose-dependently inhibited cell growth and induced apoptosis (Digestive Dis Wk (Orlando), 2003, Abs M-976). Canertinib dihydrochloride showed potent inhibition of the erbB receptor family, but this effect alone did not induce apoptosis (91st AACR (San Francisco), 2000, Abs 1533). In rats who received canertinib dihydrochloride >2.5mg/kg po, the primary toxicity was skin toxicity, with involvement of the epidermis and hair follicle. Canertinib dihydrochloride >2.5mg/kg resulted in epithelial atrophy of the gastrointestinal tract and vagina, and hepatic cholestasis (97th AACR (Washington, DC), 2006, Abs 1306). Updated by JW on 21/4/2006. - Therapeutic Activity
- Anticancer, other (K6Z)
- Pharmacological Activity
- ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
ErbB-1 tyrosine kinase inhibitor (KI-TYE1-AN)
ErbB-4 tyrosine kinase inhibitor (KI-TYE4-AN)
Epidermal growth factor receptor 3 inhibitor (GF-EPR3-AN) - Indications
- Cancer, lung, non-small cell
Cancer, colorectal
Cancer, head and neck
Cancer, thyroid
Cancer, breast
Cancer, mesothelioma
Cancer, sarcoma, general
Cancer, melanoma - Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status K6Z KI-TYE2-AN KI-TYE1-AN GF-EPR3-AN Discontinued - Indication Status
Indication Status Cancer, lung, non-small cell Discontinued Cancer, colorectal Discontinued Cancer, head and neck Discontinued Cancer, thyroid Discontinued Cancer, breast Discontinued Cancer, mesothelioma Discontinued Cancer, sarcoma, general Discontinued Cancer, melanoma Discontinued - Chemical Formula
- N - [4 - (3 - (Chloro - 4 - fluoro - phenylamino) - 7 - (3 - morpholin - 4 - yl - propoxy) - quinazolin - 6 - yl] - acrylamide dihydrochloride
- CAS Number
- 289499-45-2
- Regional Status
- USA - Discontinued
- Ratings
- Novelty: 0 - Not available
Market Size: 3 - US$ 2001-5000 million
Development Speed: 0 - Not available
Total: 0 - Update History
Date Detail 2006-04-21 Discontinued Products (Phase II Clinical Trial, Adverse events) 2004-10-06 New Indication (Cancer, various) 2002-05-31 Names Granted (CI-1033) 2001-12-18 Change in Status (Phase II Clinical Trial) 2001-12-18 New Indication (Cancer, nsclc) 2000-12-12 Change in Status (Clinical Trial) 2000-04-04 New Product in Pharmaprojects 2000-04-04 New Chemical Structure (New)
PJB Pharmaprojects Accession Number: 30640
Last Update: 2006-04-21
Originator: Pfizer (USA)
World Status: Discontinued
Origin of Material: Chemical, synthetic (CH-SY)
Route of Administration: Alimentary, po (A-PO); Parenteral, intravenous (P-IV)
Copyright PJB Publications, Richmond, Surrey, UK
| Publisher Version | Back to chart | Next record |
-
HER-2/EGFR antagonists, SUGEN/Baxter Oncology
- Investigational Drugs Database 11271
- Companies
- ASTA Medica AG (Evonik Industries AG): Licensee for development and marketing
SUGEN Inc (Pfizer Inc): Originator developing and marketing own product
Baxter Oncology GmbH (Baxter International Inc): Licensee for development and marketing - Development Status
Company Country Status Indication Date Confidence ASTA Medica AG Germany Discontinued Breast tumor 2001-11-01 ASTA Medica AG Germany Discontinued Ovary tumor 2001-11-01 Baxter Oncology GmbH Germany No Development Reported Breast tumor 2004-08-19 Baxter Oncology GmbH Germany No Development Reported Ovary tumor 2004-08-19 SUGEN Inc US No Development Reported Breast tumor 2003-04-16 SUGEN Inc US No Development Reported Lung tumor 2003-04-16 SUGEN Inc US No Development Reported Ovary tumor 2003-04-16 SUGEN Inc US No Development Reported Prostate tumor 2003-04-16 SUGEN Inc US No Development Reported Psoriasis 2003-04-16 SUGEN Inc US No Development Reported Stomach tumor 2003-04-16
Status: No Development Reported- Summary
- SUGEN, in collaboration with Baxter Oncology (formerly ASTA Medica), was evaluating small molecule inhibitors of the receptor tyrosine kinases (TK) human EGF receptor (HER)-2 and EGFR for the potential treatment of cancer and psoriasis, including lead compound SU-11464 (D-69491) [446832], [454311]. By January 1998, SUGEN and ASTA Medica had planned to move into clinical studies early that year [274879]. However, by December 2000, the lead compound was still in preclinical testing [424791]. Preclinical data were published by Baxter in November 2002 [477053]; however, no further development has since been reported by Baxter. In April 2003, SUGEN was taken over by Pfizer [486179]; since that time no development has been reported.
- Latest Change
- 2005-06-21: one or more development status entries have been updated
(D-69491, SU-11464, EGF receptor tyrosine kinase inhibitors, SUGEN, EGFR antagonists, SUGEN/Baxter Oncology, HER-2 antagonists, SUGEN/ASTA Medica, IDDB11271, Pan-HER)

Update Date: 2005-06-21
http://www.thomson-pharma.com/report/drug?dr=11271&drname=HER-2/EGFR antagonists, SUGEN/Baxter Oncology
Indication: Stomach tumor; Prostate tumor; Psoriasis; Breast tumor; Lung tumor; Ovary tumor
Action: Anticancer; Erbb2 tyrosine kinase receptor inhibitor; Epidermal growth factor antagonist
Copyright Thomson Scientific
| Publisher Version | Back to chart | Previous record | Next record |
-
D-69491
- Development Status
- Development Status - Launched, Registered or Under Active Development
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Preclinical Baxter Oncology Cancer Preclinical Sugen Cancer - Therapeutic Class
- Oncolytic Drugs
- Condition
- Cancer
- Action
- HER2 (erbB2) Inhibitors; Inhibitors of Signal Transduction Pathways
- Related Patents
- WO 2001046196
(SU-11464)
Prous Accession Number: 324204

Registry Number: 346599-65-3 (hydrochloride)
Molecular Formula: C25H25ClFN7O3
Molecular Weight: 525.9625
Originator: Sugen
Licensee: Baxter Oncology
Highest Phase: Preclinical
Copyright Prous Science
| Back to chart | Previous record | Next record |
-
D-69491
(SU-11464)
- Company Status
Company Status Baxter International No Development Reported Pfizer No Development Reported - Summary
- D-69491 (SU11464) is a small-molecule HER2 inhibitor, which was under development by Baxter and SUGEN (Pfizer (Pharmacia before the merger)) for the treatment of tumours with high expression of HER2, such as breast, ovarian, lung and pancreatic carcinomas.
Preclinical
In vivo characterization was ongoing. In vitro, it was active in the nM range (Company Web Page, Baxter, 31 May 2002). Entered by CH on 31/5/2002. - Therapeutic Activity
- Anticancer, other (K6Z)
- Pharmacological Activity
- ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
- Indications
- Cancer, general
- Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status K6Z KI-TYE2-AN No Development Reported - Indication Status
Indication Status Cancer, general No Development Reported - Regional Status
- USA - Preclinical
- Ratings
- Novelty: 0 - Not available
Market Size: 3 - US$ 2001-5000 million
Development Speed: 0 - Not available
Total: 0 - Update History
Date Detail 2004-08-05 No Development Reported 2002-05-31 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 34181
Last Update: 2004-08-05
Originator: Baxter International (USA)
Licensee: Pfizer (USA)
World Status: No Development Reported
Origin of Material: Chemical, synthetic (CH-SY)
Route of Administration: Unknown (UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record |
-
D 69491
- IMS R&D Focus Accession Number: 2019197
- Patent Summary
- Patent search conducted - no relevant patent identified (APRIL 2007).
- Latest Information
- 8 March 2004: Further development of D 69491, a HER2 receptor tyrosine kinase inhibitor, has been discontinued by Baxter and SUGEN. The agent was undergoing preclinical studies in Germany and the USA for the potential treatment of tumors that overexpress HER2 receptors such as breast, ovarian, lung and pancreatic carcinoma.17 April 2006: Chemical data added.
- Commercial Summary
- D 69491, a HER2 receptor tyrosine kinase inhibitor, was being developed under a collaboration between Baxter and SUGEN for the treatment of cancer. However, further development has been discontinued (SUGEN, APR 2003). The agent, was being developed for use in the treatment of tumors that overexpress HER2 receptors such as breast, ovarian, lung and pancreatic carcinoma (Baxter, OCT 2002).
- Scientific Summary
- D 69491 had nanomolar affinity for the HER2 receptor tyrosine kinase and showed specificity for this enzyme versus the epidermal growth factor, fibroblast growth factor and platelet derived growth factor receptor tyrosine kinases (Baxter, OCT 2002).
- Indications
- cancer
- Therapeutic Class
- All Other Antineoplastics (L1X9)
- Mechanism of Action
- signal transduction inhibitor
tyrosine kinase inhibitor - Indication Status
Country Indication Phase Germany cancer Discontinued USA cancer Discontinued - Franchise
Company Parent Relation Region Baxter Baxter co-developer SUGEN Pfizer co-developer - Update History
Date Detail DATE UNKNOWN Discontinued. OCT 2002 Preclinical, Germany, USA (cancer).
Publication Date: 2007-01-01
Originator: Baxter (USA); SUGEN (USA)
Highest Phase: Discontinued
Chemical Name: unspecified
CAS Number: 845681-48-3 (D 69491)
Copyright IMS World Publications
| Publisher Version | Back to chart | Next record |
-
Research programme: anti-HER2 monoclonal antibodies - InNexus Biotechnology
(DXL 702, DXL702)
- Drug Development Phase
Indication Phase Country Breast cancer Preclinical Canada - Properties
- Mechanism Of Action: HER2 inhibitors
Route of Administration: Parenteral - Commercial Introduction
- InNexus Biotechnology is using its proprietary DXL[trademark] technology to develop humanised monoclonal antibodies targeted at the HER2 receptor, for the treatment of breast cancer. The DXL[trademark] technology aims to improve the efficacy of second generation humanised monoclonal antibody products by increasing the binding affinity to antigen targets, enhancing antibody effector functions and conferring additional properties, such as the ability to trigger apoptosis.
The lead antibody from this programme, DXL 702, is in preclinical trials in Canada. InNexus plans to file an IND application for the compound in the US<REF href='adis://adnm/809085437' />. - Drug Development History
Date Event 21 Feb 2008 New Profile 21 Feb 2008 Preclinical trials in Breast cancer in Canada (Parenteral)
Last Update: 2008-02-21
Adis R&D Insight accesion number: 800027823
Indications: Breast cancer
Therapeutic Class (WHO): Monoclonal antibodies (L01X-C)
Therapeutic Class (EphMRA): Antineoplastic monoclonal antibodies (L1X3)
Originator: InNexus Biotechnology
Highest Phase: Preclinical
© 2008 Adis Data Information BV
| Publisher Version | Back to chart | Previous record | Next record |
-
DXL-702
- Development Status
- Development Status - Launched, Registered or Under Active Development
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Preclinical InNexus Biotechnology Cancer, breast - Therapeutic Class
- Breast Cancer Therapy
- Condition
- Cancer, breast
- Action
- Anti-HER2/neu/ErbB2
Prous Accession Number: 471338
Originator: InNexus Biotechnology
Highest Phase: Preclinical
Copyright Prous Science
| Back to chart | Previous record | Next record |
-
DXL-702
- Company Status
Company Status InNexus Biotechnology Preclinical - Summary
- DXL-702 is a MAb specific for Her2/neu, under development by InNexus Biotechnology for the treatment of breast cancer. It uses InNexus' Dynamic Cross Linking technology.
Clinical
Phase I
An IND filing is planned (Press release, InNexus, 19 Feb 2008). Entered by RV on 21/2/2008. - Therapeutic Activity
- Monoclonal antibody, other (T3A9)
Anticancer, immunological (K3) - Pharmacological Activity
- Epidermal growth factor receptor 2 antagonist (GF-EPR2-AN)
- Indications
- Cancer, breast
- Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status T3A9 GF-EPR2-AN Preclinical K3 GF-EPR2-AN Preclinical - Indication Status
Indication Status Cancer, breast Preclinical - Regional Status
- USA - Preclinical
- Ratings
- Novelty: 1 - All Preclinical
Market Size: 3 - US$ 2001-5000 million
Development Speed: 1 - Development not started
Total: 0 - Update History
Date Detail 2008-02-21 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 47029
Last Update: 2008-02-21
Originator: InNexus Biotechnology (USA)
World Status: Preclinical
Origin of Material: Biological, protein, antibody (BI-P-A)
Route of Administration: Parenteral, general (P-UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record | Next record |
-
MAb, HER2/neu, InNexus
(DXL 702)
- IMS R&D Focus Accession Number: 2030203
- Latest Information
- 3 March 2008: InNexus announced on 19 February 2008 that it has selected a second candidate, DXL 702, to enter preclinical evaluation from the company's program to develop monoclonal antibodies based on its Dynamic Cross Linking (DXL) technology. DXL 702 is directed against HER2/neu and is being developed for the potential treatment of breast cancer. The DXL technology integrates self-binding peptides into monoclonal antibodies, potentiating target binding. The DXL- modified antibodies cluster upon binding to the target receptor, resulting in a greater molecular mass at the point of association. This potentially facilitates cancer cell destruction by increasing apoptosis, antibody-dependent cytotoxicity and complement-dependent cytotoxicity. DXL modification can also extend the dissociation rate of these antibodies.
- Commercial Summary
- InNexus is developing DXL 702, a humanized monoclonal antibody directed against HER2/neu that has been enhanced using InNexus' Dynamic Cross Linking (DXL) technology, for the potential treatment of breast cancer. The DXL technology integrates self-binding peptides into monoclonal antibodies, potentiating target binding. The DXL- modified antibodies cluster upon binding to the target receptor, resulting in a greater molecular mass at the point of association. This potentially facilitates cancer cell destruction by increasing apoptosis, antibody-dependent cytotoxicity and complement-dependent cytotoxicity. DXL modification can also significantly extend the dissociation rate of the antibody. Preclinical studies are ongoing in the USA (InNexus, FEB 2008).
- Scientific Summary
- No data available.
- Indications
- cancer
solid tumor
breast cancer - Therapeutic Class
- Antineoplastic Monoclonal Antibodies (L1X3)
- Mechanism of Action
- biotechnology
protein
monoclonal antibody - Indication Status
Country Indication Phase USA cancer Preclinical USA solid tumor Preclinical USA breast cancer Preclinical - Franchise
Company Parent Relation Region InNexus InNexus developer - Update History
Date Detail FEB 2008 Preclinical, USA.
Publication Date: 2008-02-29
Originator: InNexus (USA)
Highest Phase: Preclinical
Copyright IMS World Publications
| Back to chart | Previous record |
-
MAb, Her2/neu, InNexus
(MAb, ErbB2, InNexus)
- IMS R&D Focus Accession Number: 2029173
- Latest Information
- 20 August 2007: InNexus is developing an anti-Her2/neu monoclonal antibody that is enhanced with InNexus' Dynamic Cross Linked (DXL) technology, for the treatment of cancer. The DXL technology integrates self-binding peptides into monoclonal antibodies, potentiating target binding and enhancing critical performance antibody factors. In preclinical studies, the anti-Her2/neu antibody was more potent compared with the parent antibody in slowing tumor progression in a xenograft model.
- Commercial Summary
- InNexus is developing an anti-Her2/neu monoclonal antibody that is enhanced with InNexus' Dynamic Cross Linked (DXL) technology, for the treatment of cancer. The DXL technology integrates self-binding peptides into monoclonal antibodies, potentiating target binding. The DXL-modified antibodies cluster upon binding to the target receptor, resulting in a greater molecular mass at the point of association. This potentially facilitates cancer cell destruction by increasing apoptosis, antibody-dependent cytotoxicity and complement-dependent cytotoxicity. DXL modification can also significantly extend the dissociation rate of the antibody. Preclinical studies are ongoing in the USA (InNexus, AUG 2007).
Preclinical data
In preclinical studies, the anti-Her2/neu antibody was more potent compared with the parent antibody in slowing tumor progression in a xenograft model (InNexus, AUG 2007). - Indications
- cancer
- Therapeutic Class
- Antineoplastic Monoclonal Antibodies (L1X3)
- Mechanism of Action
- biotechnology
monoclonal antibody
protein - Indication Status
Country Indication Phase USA cancer Preclinical - Franchise
Company Parent Relation Region InNexus InNexus developer - Update History
Date Detail AUG 2007 Preclinical, USA.
Publication Date: 2007-01-01
Originator: InNexus (USA)
Highest Phase: Preclinical
Copyright IMS World Publications
| Publisher Version | Back to chart | Next record |
-
E75
- Development Status
- Development Status - Launched, Registered or Under Active Development
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation United States Phase II Apthera Cancer, breast Prevention of recurrence high-risk breast cancer ( E75 vaccine with sargramostim [GM-CSF]) intradermal Phase I/II Apthera Cancer, prostate Treatment for androgen-negative HEr2-positive prostate cancer Phase II Apthera Cancer, breast Patients with tumors that overexpress HER2/neu and after herceptin use Phase II University of Washington Cancer, breast Patients with stage IV breast cancer and have been treated with herceptin
Development Status - No Development ReportedCountry/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation United States Phase I/II GlaxoSmithKline Cancer, breast United States Phase II GlaxoSmithKline Cancer, breast metastatic 1st line or 2nd line treatment after herceptin treatment Phase I/II Corixa Cancer, prostate Phase I/II GlaxoSmithKline Cancer, prostate - Therapeutic Class
- Prostate Cancer Therapy; Breast Cancer Therapy
- Condition
- Cancer, breast metastatic; Cancer, prostate; Cancer, breast
- Action
- Anti-HER2/neu/ErbB2
- Product Milestone History
Product Milestone History Milestone Date Milestone Condition Notes Organization Area Feb, 2003 Phase I Cancer, breast Corixa Jan, 2003 Licensed Cancer, breast Corixa Product Milestone History(Scheduled) Milestone Date Milestone Condition Notes Organization Area first half 2008 Phase II/III Cancer, breast Prevention of recurrence high-risk breast cancer ( E75 vaccine with sargramostim [GM-CSF]); Intradermal Apthera US - Related Patents
- US 2003064916; US 5876712; US 6514942; WO 1996018409
(Neuvax)
Prous Accession Number: 302089

Registry Number: 160212-35-1
Molecular Formula: C50H78N10O11
Molecular Weight: 995.2151
Originator: Henry M. Jackson Foundation; University of Washington
Licensee: GlaxoSmithKline; Corixa; Apthera
Highest Phase: Phase II
Copyright Prous Science
| Back to chart | Previous record | Next record |
-
E-75
(APT-101, NeuVax)
- Company Status
Company Status Apthera Phase III Clinical Trial - Summary
- E-75 is an immunostimulatory peptide combined with GM-CSF, under development by Apthera as a vaccine for the prevention of recurrence of HER2/NEU-expressing cancers.
Marketing
Apthera exclusively licensed E-75 from the Henry M Jackson Foundation for the Advancement of Military Medicine, MD, the US, and another major academic institution (9th Ann C21 BioVentures (Monterey), 2007).
Clinical
Phase III
It is in a randomized, pivotal Phase III registration trial in early-stage HER2+ breast cancer (15th BioPartner Eur (London), 2007). A Phase III trial in high-risk, hormone-resistant HER2-positive prostate cancer is planned for 2008, with further potential studies in ovarian, lung, colon and pancreatic cancers (9th Ann C21 BioVentures (Monterey), 2007).
Phase II
In a Phase II trial in 200 high-risk breast cancer patients, E-75 sc 1x/mth x 6mth demonstrated a 24mth recurrence rate of 56% cf 14.8% on control. Toxicity was minimal and mostly attributed to GM-CSF (Press release, Apthera, 14 Dec 2006; 9th Ann C21 BioVentures (Monterey) 2007). US Phase I/II trials in combination with trastuzumab (qv) are planned for 2007. It is in a Phase I/II trial in 50 patients with HER2+ prostate cancer. In 23/50 patients there were no major toxicities and significantly enhanced immune responses against HER2 were observed (Company Web Page, Apthera, 5 Jan 2007).
Phase I
A pilot study in 20 women in combination with trastuzumab (qv) is planned for 2007, with further studies of the combination planned once the dose and safety profile is established (9th Ann C21 BioVentures (Monterey), 2007). In a Phase I trial in 14 patients with stage IV breast or ovarian cancer who received escalating doses of E-75 + 250µg GM-CSF, the formulation was safe and well-tolerated with no grade 3 or 4 toxicity reported. 8/14 patients were evaluated for cytotoxic T-lymphocyte (CTL)-mediated immune response, and 4/8 patients showed CTL-mediated lytic activity, which remained for up to 12mth following completion of the trial.
Preclinical
E-75 + trastuzumab showed potent antitumour synergy in animal models (Company Web Page, Apthera, 5 Jan 2007).
Licensing
It is available for licensing worldwide (15th BioPartner Eur (London), 2007). Updated by JS on 29/10/2007. - Therapeutic Activity
- Anticancer, immunological (K3)
- Pharmacological Activity
- ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
- Indications
- Cancer, breast
Cancer, prostate
Cancer, ovarian - Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status K3 KI-TYE2-AN Phase III Clinical Trial - Indication Status
Indication Status Cancer, breast Phase III Clinical Trial Cancer, prostate Phase II Clinical Trial Cancer, ovarian Phase I Clinical Trial - Regional Status
- USA - Phase III Clinical Trial
- Licensing Opportunities
- Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Colombia, Denmark, Finland, France, Germany, Greece, Hong Kong, India, Ireland, Israel, Italy, Japan, Luxembourg, Malaysia, Mexico, Netherlands, New Zealand, Norway, Peru, Philippines, Portugal, Russian Federation, South Africa, South Korea, Spain, Sweden, Switzerland, Thailand, Turkey, UK, USA, Venezuela
- Ratings
- Novelty: 6 - Leading Compound
Market Size: 3 - US$ 2001-5000 million
Development Speed: 0 - Not available
Total: 0 - Update History
Date Detail 2007-10-29 Licensing Opportunities (Worldwide) 2007-10-29 Change in Status (Phase III Clinical Trial) 2007-01-08 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 43484
Last Update: 2007-10-29
Originator: Apthera (USA)
World Status: Phase III Clinical Trial
Origin of Material: Chemical, synthetic, peptide (CH-SY-P)
Route of Administration: Parenteral, subcutaneous (P-SC)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record | Next record |
-
vaccine, HER2/neu, Apthera
(E 75; NeuVax)
- IMS R&D Focus Accession Number: 2015909
- Latest Information
- 17 March 2008: Gail Thurston, VP Corporate and Business Development at Apthera, informed R&D Focus during an interview at the 6th Annual Bio Partnering North America, 3-5 February 2008, Vancouver, Canada, that worldwide partners are sought for the further development of E 75 (Neu Vax). In January 2008, the company submitted a Special Protocol Assessment (SPA) to the US FDA for a randomized, double-blind, placebo-controlled phase III trial of the vaccine for the prevention of recurrence in early-stage, node-positive breast cancer. The study will assess E 75 in combination with standard of care, compared with standard of care alone. Apthera expects to begin this trial during third quarter 2008. Apthera plans to begin a pivotal phase III trial of E 75 for the treatment of high-risk, hormone resistant, HER2- positive prostate cancer during 2008. The product is also being evaluated in a pilot safety study in combination with trastuzumab (HERCEPTIN) as a therapy for breast cancer. ....E 75, a vaccine comprising an immunogenic peptide derived from the HER2/neu protein, is designed to elicit persistent activation of a tumor-specific cytotoxic T-cell immune response with a once-monthly intradermal dosing schedule.
- Commercial Summary
- Apthera (formerly Advanced Peptide Therapeutics) is developing E 75 (NeuVax), a vaccine comprising an immunogenic peptide derived from the HER2/neu protein, for the prevention and treatment of solid tumors such as breast, prostate, pancreatic, ovarian and nonsmall cell lung cancers. In January 2008, Apthera submitted a special protocol assessment to the US FDA for a phase III trial of E 75 in the prevention of breast cancer recurrence in the USA. A phase I/II trial for the treatment of prostate cancer has been conducted. A phase I trial of E 74 in combination with trastuzumab for the treatment of breast cancer is under way. Advanced Peptide Therapeutics (now Apthera) acquired exclusive rights to E 75 from University of Texas MD Anderson Cancer Center (USA) and the Henry Jackson Foundation for the Advancement of Military Medicine (USA), upon its formation through an alliance with these institutes in July 2005.
R&D progress
Breast cancer
Apthera has submitted to the US FDA a special protocol assessment (SPA) for a randomized, double-blind, placebo-controlled phase III trial of E 75 in the prevention of recurrence in early-stage, node- positive breast cancer. The study will assess E 75 in combination with standard of care, compared with standard of care alone (Apthera, JAN 2008). Apthera expects this trial to begin during third quarter 2008 (Apthera, FEB 2008). Apthera plans to conduct a phase III trial of E 75 that will enroll women with node-positive, HER2-positive breast cancer and will assess the disease-free survival (DFS) at three years. Consequently, E 75 will be evaluated in node-negative breast cancer patients at high risk of recurrence, as well as in breast cancer patients with bone metastasis (Apthera, OCT 2007). A phase III trial of E 75 in the prevention of breast cancer recurrence was expected to begin second half 2007 (Apthera, FEB 2007). A phase II trial of E 75 in the prevention of breast cancer recurrence has been conducted in the USA. The trial aims to evaluate the safety and dose-optimize the vaccine in node-positive (NP) and node-negative (NN) patients. All patients had undergone standard surgery and chemotherapy and were disease-free before enrollment. Immune response was measured by vitro functional assays and in vivo DTH responses, and clinical recurrence rates were also monitored. Data have been reported after enrollment of 90 NP and 81 NN patients; 90 HLA-A2- and HLA-A3- positive patients received E75 and 81 patients with other HLA types were placed in the observation group (Apthera, DEC 2005). A phase I/II of E 75 in combination with trastuzumab for the treatment of breast cancer has been approved by the US FDA for patient accrual. The trial will assess the dose and safety profile of the combination (Apthera, OCT 2007). This pilot safety study, involving 15-20 women, has initiated (Apthera, JAN 2008).
Prostate cancer
E 75 has been evaluated in a phase I/II trial in 47 patients with hormone-refractory, HER2-positive prostate cancer who were at high risk of disease recurrence (Apthera, OCT 2007). Apthera plans to begin a pivotal phase III trial of E 75 for the treatment of high-risk, hormone resistant, HER2-positive prostate cancer during 2008 (Apthera, FEB 2008). Preliminary data have been reported from an ongoing phase I trial in the treatment of HER2-positive prostate cancer (Apthera, FEB 2007). A phase I trial has been initiated involving ten patients evaluating the E 75 HER2/neu vaccine peptide as a preventative vaccine targeting prostate cancer patients at high risk of recurrence (Walter Reed Army Institute of Research, MAR 2001).
Licensing/Partnering
Advanced Peptide Therapeutics (now Apthera) acquired exclusive rights to E 75 from University of Texas MD Anderson Cancer Center (USA) and the Henry Jackson Foundation for the Advancement of Military Medicine (USA) in July 2005 (Apthera, FEB 2007).
Availability
The E 75 program is available for partnering, worldwide (Apthera, FEB 2008).
Company predictions
Apthera expects to begin a phase III trial of E 75 in early-stage breast cancer patients during third quarter 2008. Apthera plans to begin a pivotal phase III trial of E 75 for the treatment of high- risk, hormone resistant, HER2-positive prostate cancer during 2008 (Apthera, FEB 2008). Apthera has received guidance from the US FDA regarding a special protocol assessment (SPA) for a phase III trial, the approval of which is expected by end 2007, and Apthera plans to start a phase III trial of E 75 first/second quarter of 2008. A phase I/II of E 75 in combination with trastuzumab (HERCEPTIN) has been approved by the US FDA for patient accrual. The trial will likely start by end 2007. A phase I/II trial in the treatment of ovarian and bladder cancer has been approved by Institutional Review Boards (IRBs) and is scheduled for second half 2008 (Apthera, OCT 2007). A phase III trial of E 75 in the prevention of breast cancer recurrence is expected to begin second half 2007 (Apthera, FEB 2007).
Preclinical data
Preclinical studies, involving cytotoxic T lymphocytes extracted from 12 patients with HER2/neu positive prostate cancer, show that the peptide induces an immune response against tumor cells in vitro. The CTLs stimulated with the peptide are cytotoxic to HER2/neu positive and HLA-A2 positive tumor cells (92nd AACR, Abs 3668, MAR 2001).
Clinical data
Results from a phase I pilot study showed that E 75 is immunogenic in prostate cancer patients (92nd AACR, Abs 3668, MAR 2001). In an ongoing phase I trial in the treatment of HER-2-positive prostate cancer, preliminary data indicated only minor toxicities in the 23 patients (of 50 patients enrolled) given E 75. Patients given the vaccine also achieved significantly enhanced T-cell mediated immune responses against HER2 (Apthera, FEB 2007). A phase II evaluated E 75 in the prevention of breast cancer recurrence. The trial's aim was to evaluate the safety and dose- optimize the vaccine in node-positive (NP) and node-negative (NN) patients. All patients had undergone standard surgery and chemotherapy and were disease-free before enrollment. Immune response was measured by vitro functional assays and in vivo DTH responses, and clinical recurrence rates were also monitored. Data has been assessed after enrollment of 90 NP and 81 NN patients; 90 HLA-A2- and HLA-A3-positive patients received E 75 and 81 patients with other HLA types were placed in the observation group. Combined data from the trials indicated minimal toxicity was observed, with rates of Grade I and Grade II local reactions being 86% and 14%, respectively. Rates for Grades 0, I, II and III systemic toxicity were 16%, 70%, 13% and 1%, respectively; these were mainly caused by the GM-CSF. All patients given E 75 demonstrated in vivo immunologic responses as well as in vivo DTH responses. Clinical recurrence rates at 24 months were observed in five (5.6%) and 12 (14.8%) patients in the E 75 and observation groups, respectively (p=0.04) (Apthera, DEC 2005). - Indications
- cancer
solid tumor
genitourinary cancer
prostate cancer
breast cancer
bladder cancer - Therapeutic Class
- All Other Immunostimulating Agents Excluding Interferons (L3A9)
All Other Vaccine Preparations (J7C) - Mechanism of Action
- vaccine
peptide - Indication Status
Country Indication Phase USA breast cancer Phase II USA prostate cancer Phase II - Licensing Opportunities
- Available for licensing in: Worldwide.
- Licensing Contact
- Gail Thurston,
VP Corporate and Business Development,
Apthera,
14861 N Scottsdale Road,
Suite 105,
Scottsdale,
AZ 85254,
USA;
Tel: +1 800 254 4841;
Fax: +1 480 393 7088;
Email: gthurston@apthera.com. - Franchise
Company Parent Relation Region Henry M Jackson Foundation Henry M Jackson Foundation licensor MD Anderson Cancer Center Texas University licensor Apthera Apthera licensee Worldwide Walter Reed Army Institute of Research Walter Reed Army Institute of Research other - Update History
Date Detail FEB 2008 Available for partnering, Worldwide. Q4 2007 Phase I, USA (breast cancer; in combination with trastuzumab). 1H 2007 Phase I/II, USA (prostate cancer). FEB 2007 Available for partnering, Worldwide. NOV 2006 Advanced Peptide Therapeutics changes its name to Apthera. 2006 Phase II, USA (breast cancer). JUL 2005 Agreement between Apthera and the University of Texas MDAnderson Cancer Center (USA) and the Henry M Jackson Foundation forthe Advancement of Military Medicine (USA). MAR 2001 Phase I, USA (prostate cancer).
Publication Date: 2008-03-14
Originator: Henry M Jackson Foundation (USA); MD Anderson Cancer Center (USA)
Licensee: Apthera (USA); Walter Reed Army Institute of Research (USA)
Highest Phase: Phase II
Substance Origin: vaccine; recombinant biotechnology
Copyright IMS World Publications
| Back to chart | Previous record |
-
APT 101
- IMS R&D Focus Accession Number: 2026873
- Latest Information
- 29 October 2007: Apthera is has suspended development of APT 101, an injectable nine amino acid peptide epitope derived from the extracellular domain of the HER2 tumor antigen, as an immunotherapy for HER2-expressing solid tumors, including breast, prostate, ovarian, lung and pancreatic cancer. Phase II/III trials were under way in breast cancer. Apthera will concentrate its efforts on NeuVax.
- Commercial Summary
- Apthera (formerly Advanced Peptide Therapeutics) was developing APT 101, an injectable nine amino acid peptide epitope derived from the extracellular domain of the HER2 tumor antigen, as an immunotherapy for HER2-expressing solid tumors, including breast, prostate, ovarian, lung and pancreatic cancer; however, development has been suspended due to major efforts directed at NeuVax. The peptide bound to Leukocyte Antigen (HLA class I) molecules leading to the activation and proliferation of cytotoxic T-cells. A pivotal US phase IIb/III trial of the agent in the treatment of HER2-positive stage II and III breast cancer was under way in the USA. A US phase IIb trial of APT 101 in the treatment of HER2-positive hormone-sensitive and hormone- resistant forms of prostate cancer was under way. APT 101 has been evaluated in five phase I and phase II trials; over 150 patients with breast and prostate cancer had been treated with the peptide. Advanced Peptide Therapeutics (now Apthera) acquired rights to intellectual property relating to APT 101 from the MD Anderson Cancer Center (USA) and the Henry M Jackson Foundation (USA) in July 2005.
R&D progress
All trials of APT 101 have been suspended (Apthera, OCT 2007). A pivotal US phase IIb/III trial of APT 101 in the treatment of HER2- positive stage II and III breast cancer was under way in the USA. A US phase IIb trial of APT 101 in the treatment of HER2-positive hormone- sensitive and hormone-resistant forms of prostate cancer was under way. APT 101 had been evaluated in five phase I and phase II trials; over 150 patients with breast and prostate cancer had been treated with the peptide. A US phase IIb trial of APT 101 in the treatment of HER2-positive hormone-sensitive and hormone-resistant forms of prostate cancer was under way (Advanced Peptide Therapeutics, APR 2006).
Licensing/Partnering
Advanced Peptide Therapeutics acquired rights to intellectual property relating to APT 101 from the MD Anderson Cancer Center (USA) and the Henry M Jackson Foundation (USA) in July 2005 (Advanced Peptide Therapeutics, APR 2006).
Availability
APT 101 was available for partnering, worldwide (Advanced Peptide Therapeutics, APR 2006).
Company predictions
Enrollment into a pivotal US phase IIb/III trial of APT 101 in the treatment of HER2-positive stage II and III breast cancer, was expected to complete early 2007. A phase I trial to evaluate APT 101 in combination with trastuzumab (HERCEPTIN) in advanced breast cancer patients was being planned. Advanced Peptide Therapeutics planned to meet with the US FDA in 2006 to discuss a Special Protocol Assessment (SPA) for pivotal trials of APT 101 in the treatment of HER2-positive hormone-sensitive and hormone-resistant forms of prostate cancer, are expected to begin in 2007. Phase I trials of the agent in ovarian, lung and pancreatic cancers were expected to begin first quarter 2007 (Advanced Peptide Therapeutics, APR 2006).
Clinical data
Results from phase I and phase II trials of APT 101 in patients with breast cancer and prostate cancer indicated that high level anti-HER2 tumor proliferative T-cell responses were elicited in all patients immunized with APT 101 plus adjuvant. These patients developed a DTH recall response and survived disease-free for a longer duration than patients not treated with APT 101 (Advanced Peptide Therapeutics, APR 2006). - Indications
- cancer
solid tumor
breast cancer
genitourinary cancer
ovarian cancer
prostate cancer
gastrointestinal cancer
pancreatic cancer
lung cancer - Therapeutic Class
- All Other Immunostimulating Agents Excluding Interferons (L3A9)
- Mechanism of Action
- peptide
immunostimulant - Indication Status
Country Indication Phase USA breast cancer Suspended USA prostate cancer Suspended USA ovarian cancer Suspended USA pancreatic cancer Suspended USA lung cancer Suspended - Franchise
Company Parent Relation Region Henry M Jackson Foundation Henry M Jackson Foundation licensor MD Anderson Cancer Center Texas University licensor Apthera Apthera licensee - Update History
Date Detail 2007 Suspended. NOV 2006 Advanced Peptide Therapeutics changes its name to Apthera. APR 2006 Available for partnering, Worldwide. 2H 2005 Phase II/III, USA (breast cancer). Phase IIb, USA (prostatecancer). Preclinical, USA (lung, pancreatic, ovarian cancers). JUL 2005 Agreement between Advanced Peptide Therapeutics, and the MDAnderson Cancer Center (USA) and the Henry M Jackson Foundation (USA).
Publication Date: 2007-10-26
Originator: Henry M Jackson Foundation (USA); MD Anderson Cancer Center (USA)
Licensee: Apthera (USA)
Highest Phase: Suspended
Copyright IMS World Publications
| Publisher Version | Back to chart |
-
erbB2 tyrosine kinase receptor inhibitor (cancer), AstraZeneca
- Investigational Drugs Database 56969
- Companies
- AstraZeneca plc: Originator developing and marketing own product
- Development Status
Company Country Status Indication Date Confidence AstraZeneca plc UK Discovery Cancer 2005-05-06
Status: Discovery- Summary
- AstraZeneca is investigating a series of erbb2 receptor tyrosine kinase inhibitors, including a lead compound (structure shown) which also exhibits anti-EGFR activity, for the potential treatment of cancer. Preclinical work was ongoing in October 2007 [842147].
- Latest Change
- 2008-03-07: 1 Reference Added [883650]
(erbB2 kinase inhibitors (cancer), AstraZeneca, IDDBCP188446, IDDBCP199515)

Update Date: 2008-03-07
http://www.thomson-pharma.com/report/drug?dr=56969&drname=erbB2 tyrosine kinase receptor inhibitor (cancer), AstraZeneca
Indication: Cancer
Action: Anticancer; Erbb2 tyrosine kinase receptor inhibitor; Epidermal growth factor antagonist
Copyright Thomson Scientific
| Publisher Version | Back to chart | Next record |
-
HER-2 protein AutoVac
- Investigational Drugs Database 45246
- Companies
- Pharmexa A/S: Originator developing and marketing own product
- Development Status
Company Country Status Indication Date Confidence Pharmexa A/S Denmark Discovery Breast tumor 2000-05-25 Pharmexa A/S Hungary Discontinued Breast tumor 2006-08-14 Pharmexa A/S Poland Phase 2 Clinical Breast tumor 2005-01-25 Pharmexa A/S Romania Phase 2 Clinical Breast tumor 2005-12-15 Pharmexa A/S Russian Federation Phase 2 Clinical Breast tumor 2005-12-15 Pharmexa A/S Denmark Discovery Ovary tumor 2000-05-25
Status: Phase 2 Clinical- Summary
- Pharmexa (formerly M&E Biotech) is developing a therapeutic vaccine, HER-2 protein AutoVac (PX-104.1), for the im potential treatment of breast and ovarian cancers [368282], [679139]. In January 2005, Pharmexa initiated a phase II trial with the vaccine formulated with aluminum hydroxide (Alhydrogel) as the adjuvant; results were expected to be available by mid-2006 [581683]. However, by August 2006, this study had been discontinued, following an analysis of preliminary data [684131]. In December 2005, Pharmexa initiated a second phase II trial in Poland, Russia and Romania [641118]. By April 2005, Pharmexa was seeking to outlicense the vaccine by 2006 [594584]; however, the drug was still listed on the company's pipeline in March 2006 [676565].
- Latest Change
- 2006-08-15: 1 reference added [684131]
(ME-104, PX-104.1, HER-2 protein pharmaccine, M&E Biotech, HER-2 protein pharmaccine, Pharmexa, HER-2 protein vaccine (cancer), Pharmexa)
Update Date: 2006-08-24
http://www.thomson-pharma.com/report/drug?dr=45246&drname=HER-2 protein AutoVac
Indication: Breast tumor; Ovary tumor
Action: Therapeutic vaccine; Anticancer; Erbb2 tyrosine kinase receptor inhibitor; Epidermal growth factor antagonist
Technology: Intramuscular formulation; Protein (recombinant); Tumor antigen
Copyright Thomson Scientific
| Publisher Version | Back to chart | Previous record | Next record |
-
HER-2 Protein AutoVac
- Development Status
- Development Status - No Development Reported
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Europe Discontinued Pharmexa Cancer, breast metastatic parenteral In combination with QS-21 adjuvant Europe Phase II Pharmexa Cancer, breast metastatic parenteral Formulation in Alhydrogel adjuvant - Therapeutic Class
- Breast Cancer Therapy
- Condition
- Cancer, breast metastatic
- Action
- Anti-HER2/neu/ErbB2
- Summary
- PX-104.1 is a HER-2 protein vaccine in clinical development for the treatment of cancer. Phase II clinical trials had been under way at the company with PX-104.1 in combination with the adjuvant QS-21, a proprietary adjuvant licensed to Pharmexa from Antigenics in January 2005, for the treatment of metastatic breast cancer, however, the trials were discontinued based on a review of preliminary data.The PX-104.1 molecule is a HER-2 antigen, modified to include two promiscuous and highly immunogenic peptides derived from tetanus toxin and is designed to overcome tolerance to HER-2 and induce effective anti-HER-2 polyclonal antibody responses in breast cancer patients where HER-2 is overexpressed.
- Product Milestone History
Product Milestone History Milestone Date Milestone Condition Notes Organization Area Aug 14, 2006 Discontinued Cancer, breast metastatic Discontinued by Pharmexa for the treatment of metastatic breast cancer, in combination with the adjuvant QS-21; Injections.The company is disappointed with the preliminary results and will investigate other routes for the product Pharmexa Europe Nov 11, 2004 Phase II Cancer, breast Injections, formulations using either Alhydrogel or QS-21 adjuvants Pharmexa Europe Sep 10, 2003 Phase I Cancer, breast Injections Pharmexa US Mar 31, 2003 IND Filed Cancer, breast Pharmexa US - Related Patents
- WO 1995005849
(PX-104.1.6; ME-104; PX-104.1)
Prous Accession Number: 319773
Originator: Pharmexa
Highest Phase: Discontinued
Copyright Prous Science
| Back to chart | Previous record |
-
PX-104.1
(anti-HER-2 protein, Pharmexa, HER-2 protein AutoVac,Pharmacc, ME-104)
- Company Status
Company Status Pharmexa Phase II Clinical Trial - Summary
- PX-104.1 is an anti-HER-2 protein Pharmaccine, under development by Pharmexa (previously M&E Biotech) for the treatment of breast cancer. It is part of a collaboration with CRC Laboratories, London, the UK. ME-103 (qv) was also under development as part of the collaboration (Direct communication, M&E Biotech, 9 Jan 2001). Development was previously reported as discontinued due to lack of efficacy, but Pharmexa continues to evaluate it (Scrip Daily Online, 14 Aug 2006, S00930364; Ann Rep, Pharmexa, 2007).
Marketing
Pharmexa hold worldwide rights to PX-104.1 (Ann Rep, Pharmexa, 2007). GlaxoSmithKline (GSK) had an exclusive option to license the project after Phase I completion, as part of an agreement whereby GSK licensed a vector-cell production system to Pharmexa. Pharmexa had a 2yr manufacturing, development and clinical supply agreement with Xenova (now Celtic Pharma) for contract manufacture of the vaccine for Phase II trials. Pharmexa had a licensing agreement with Antigenics to use its vaccine adjuvant, QS-21 (qv) with PX-104.1 (Scrip Daily Online, 18 Jan 2005, S00869760).
Clinical
Phase II
In a Phase II trial in Hungary and Poland in 40 metastatic breast cancer patients, receiving PX-104.1 1.25mg x4 x6wk followed by booster immunizations q 4wk, until patients showed disease progression, preliminary results from 10/40 patients showed that the primary endpoint would not be met. 6/7 patients who received 4 doses by wk8 showed desired levels of antibodies to the HER-2 receptor, demonstrating biological activity and immunogenicity. No serious adverse events were reported and secondary endpoints (immune response and safety) were met (Scrip Daily Online, 18 Jan 2005, S00869760 & 14 Aug 2006, S00930364; Ann Rep, Pharmexa, 2007). A 2nd Phase II trial in Hungary and Poland for breast cancer, using QS-21 as an adjuvant to PX-104.1 in up to 50 patients, was planned (Scrip Daily Online, 18 Jan 2005, S00869760).
Phase I
In a US Phase I trial, PX-104.1 x4 over 10wk led to antibody responses to HER-2 in 6/10 patients with advanced HER-2-positive breast cancer. The 1st responses were detected after 2 injections and were significantly boosted following subsequent immunizations. The peak antibody concentration detected in patient sera was approximately 2µg/ml, and the average for all 6 responders was 0.8µg/ml. The responses declined after treatment had stopped. No serious adverse events were reported (Press releases, Pharmexa, 19 Jan & 17 Mar 2004).
Preclinical
In monkeys, 2 low-dose PX-104.1 injections induced therapeutic antibody reactions comparable to trastuzumab (qv) (Direct communication, Pharmexa, 1 Oct 2002). In a pilot study, groups of 6 monkeys receiving either PX-104.1 100mg formulated with 1 of 2 adjuvants (A or B) or a standard aluminium adjuvant either x5 q 2wk or x3 at wk0, 2 and 6, the highest response was seen with PX-104.1 + adjuvant A x5 q 2wk. Similar responses were seen with PX-104.1 + adjuvant A or aluminium x3 at wk0, 2 and 6. In a 3-9mth repeated dose study with monkeys receiving PX-104.1 20, 100 or 500mg at wk0 and 2 and then q 4wk x6mth, a significant antibody response was observed in the 500mg group after 1 immunization. In the 20 and 100mg groups, titres developed after 2 immunizations. Titres were maintained by continued boosting and declined rapidly in the recovery period. No vaccine-associated toxicity was observed (12th Eur Can Conf (Copenhagen), 2003, Abs 969). Updated by JS on 27/3/2008. - Therapeutic Activity
- Recombinant vaccine (T2B)
Anticancer, immunological (K3) - Pharmacological Activity
- ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
Immunostimulant (IM-AG) - Indications
- Cancer, breast
- Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status T2B KI-TYE2-AN IM-AG Phase II Clinical Trial K3 KI-TYE2-AN IM-AG Phase II Clinical Trial - Indication Status
Indication Status Cancer, breast Phase II Clinical Trial - Regional Status
- Denmark - Phase II Clinical Trial
UK - Preclinical
USA - Phase I Clinical Trial - Ratings
- Novelty: 6 - Leading Compound
Market Size: 3 - US$ 2001-5000 million
Development Speed: 4 - Faster than Average
Total: 13 - Update History
Date Detail 2008-03-27 Development Continuing 2006-08-14 Discontinued Products (Phase II Clinical Trial, Efficacy) 2005-01-18 Change in Status (Phase II Clinical Trial) 2005-01-18 New Licensees (Antigenics, Unspecified countries) 2003-08-21 Change in Status (Phase I Clinical Trial) 2000-11-28 Compounds Identified (anti-breast cancer vaccine, M&E) 1998-01-15 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 26826
Last Update: 2008-03-27
Originator: Pharmexa (Denmark)
World Status: Phase II Clinical Trial
Origin of Material: Biological, protein, recombinant (BI-P-R)
Route of Administration: Parenteral, general (P-UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Publisher Version | Back to chart |
-
HKI-357
- Investigational Drugs Database 60268
- Companies
- Wyeth: Originator developing and marketing own product
- Development Status
Company Country Status Indication Date Confidence Wyeth US Discovery Cancer 2000-09-04
Status: Discovery- Summary
- Wyeth is investigating HKI-357, a lead in a series of HER2 receptor tyrosine kinase inhibitors, for the potential oral treatment of cancer. From June 2004 to June 2005, Wyeth listed HKI-357 as being in preclinical studies [ 541985], [ 580331]. The compound was not listed on Wyeth's pipeline in February 2006 [ 737969] but re-appeared on the pipeline as being in preclinical studies in March 2008 [ 905133].
- Latest Change
- 2008-05-28: Minor Editorial Amendment
(CPD-820, WAY-177820, compound 820, Wyeth-Ayerst, EGF-R kinase inhibitors (oral, cancer), Wyeth-Ayerst, erb-B2 inhibitors (oral, cancer), Wyeth-Ayerst, HER-2 inhibitors (oral, cancer), Wyeth, HER-2 inhibitors (oral, cancer), Wyeth-Ayerst, tyrosine kinase inhibitors (oral, cancer), Wyeth-Ayerst)
CAS Registry No.: 214484-33-0

Update Date: 2008-05-28
http://www.thomson-pharma.com/report/drug?dr=60268&drname=HKI-357
Indication: Cancer
Action: Anticancer; Erbb2 tyrosine kinase receptor inhibitor; Epidermal growth factor antagonist
Technology: Capsule formulation
Copyright Thomson Scientific
| Publisher Version | Back to chart | Next record |
-
MDX H210
(MDX-H210)
- Drug Development Phase
Indication Phase Country Prostate cancer Discontinued(II) USA Breast cancer Discontinued(I) USA - Properties
- Mechanism Of Action: HER2 inhibitors
Pharmacodynamics: Induces TNF-alpha in prostate cancer patients and stabilises PSA levels; reduces HER-2 levels
Route of Administration: IV-infusion
Adverse Events: ; Occasional: Chest pain, Chills, Fever, Nausea, Thrombocytopenia, Vomiting - Commercial Introduction
- MDX H210 is a bispecific half humanised antibody that consists of monoclonal antibody H22 that binds the high affinity receptor Fcgamma R1, and monoclonal antibody 520C9 that recognises the erb-2 oncoprotein (HER-2). The HER-2 receptor is often overexpressed in a number of different cancer types including prostate, kidney, colon and breast cancers. MDX H210 directs and activates effector cells, including monocytes, macrophages and activated (by cytokines) neutrophils.
Medarex was developing MDX H210 in the US for the treatment of HER-2 expressing cancers. The agent was undergoing phase II trials in patients with prostate cancer and phase I studies in breast cancer. However, it appears that development of this compound has been discontinued. - Review
- Pharmacokinetics
- MDX H210 produced plasma concentrations associated with clinical activity in patients with HER2/neu-overexpressing prostate cancer. Evidence of immunological activity was observed, with up to 70% saturation of peripheral blood monocyte FcgammaRI at 1h after infusion at the 4 and 8 mg/m2 dose levels, and decreased plasma concentrations of HER2/neu<REF href='adis://adnm/800859972' />.
- Pharmacodynamics
- Prostate cancer: induction of low concentrations of tumour necrosis factor alpha and interleukin-6 in blood plasma were observed immediately after MDX H210 infusion to prostate cancer patients. MDX H210 induced a 70% saturation of circulating monocyte-associated FcgammaR1 at dose levels in the range of 4-8 mg/m2. Five of 6 patients achieved stable levels of prostate specific antigen for >= 40days. Circulating plasma levels of HER-2 decreased by 80% at days 12 and 29 with MDX H210 treatment<REF href='adis://adnm/800859972' />.
- Therapeutic Trials
- Prostate cancer: ongoing phase II clinical trials showed that treatment with MDX H210 resulted in quality of life and improvements in prostate specific antigen (PSA) levels in patients with advanced prostate cancer. Nine of 22 patients experienced a reduction of serum levels of PSA. Seven of the patients had PSA reductions of 50-99%. 40% of patients evaluated for quality of life reported a significant reduction in average pain levels after MDX H210 treatment<REF href='adis://adnm/800688013' />.
Five of the 18 patients with prostate cancer displayed an objective response as classified by a > 50% reduction in prostate-specific antibody<REF href='adis://adnm/800697040' />.
MDX H210 stabilised prostate-specific antigen levels in 5/6 evaluable patients with HER2/neu-overexpressing prostate cancer. The mean survival duration was 16.5 months<REF href='adis://adnm/800859972' />.
The bispecific antibody MDX-H210 in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), had activity against advanced, hormone-refractory HER2-positive prostate cancer. In this study, 25 patients with hormone-refractory HER2-positive adenocarcinoma of the prostate received treatment cycles comprising SC GM-CSF 5 ?g/kg/day for 4 days plus IV MDX-H210 15 mg/m2 on day 4, repeated weekly for 6 weeks. Among the 20 patients were evaluable for efficacy, reductions in prostate specific antigen (PSA) levels of > 50% were seen in 35% of these patients; the reductions ranged from 51 to 99% and the durations of the reductions were 71 to 184+ days. In addition, 50% of the 12 patients with evaluable pain had improvements in pain scores; all of these patients had PSA reductions of > 25%. Responses lasted for 1-12 cycles<REF href='adis://adnm/800877620' />.
Renal cancer: ongoing phase II clinical trials showed that treatment with MDX 210 resulted in tumour reduction and disease stabilisation in patients with renal cancer who had failed standard therapy. 13/20 patients experienced disease stabilisation, including 2 who had tumour reductions of >= 49%<REF href='adis://adnm/800688013' />.
A phase II trial combining MDX 210 and granulocyte-macrophage colony-stimulating factor in 23 patients with advanced prostate and renal cell carcinomas has been completed, with 2/5 patients with renal cancer having an objective response<REF href='adis://adnm/800697040' />. - Drug Development History
Date Event 03 May 2007 Discontinued - Phase-I for Breast cancer in USA (IV-infusion) 03 May 2007 Discontinued - Phase-II for Prostate cancer in USA (IV-infusion) 12 Sep 2001 A clinical study has been added to the Cancer therapeutic trials section 20 Mar 2001 New profile 20 Mar 2001 Phase-I clinical trials for Breast cancer in USA (IV-infusion) 20 Mar 2001 Phase-II clinical trials for Prostate cancer in USA (IV-infusion)
Last Update: 2007-05-03
Adis R&D Insight accesion number: 800015467
Indications: Breast cancer; Prostate cancer
Therapeutic Class (WHO): Monoclonal antibodies (L01X-C)
Therapeutic Class (EphMRA): All Other Antineoplastic (L1X)
Originator: Medarex
Highest Phase: Discontinued II
© 2008 Adis Data Information BV
| Publisher Version | Back to chart | Previous record | Next record |
-
IDM-1
- Investigational Drugs Database 14401
- Companies
- Immuno-Designed Molecules SA (IDM Pharma Inc): Originator developing and marketing own product
- Development Status
Company Country Status Indication Date Confidence Immuno-Designed Molecules SA Australia No Development Reported Ovary tumor 2005-06-13 Immuno-Designed Molecules SA Belgium No Development Reported Ovary tumor 2005-06-13 Immuno-Designed Molecules SA Canada No Development Reported Ovary tumor 2005-06-13 Immuno-Designed Molecules SA France No Development Reported Ovary tumor 2005-06-13 Immuno-Designed Molecules SA Western Europe No Development Reported Ovary tumor 2005-06-13
Status: No Development Reported- Summary
- Immuno-Designed Molecules (IDM) was developing IDM-1 (Osidem), a combination of Medarex's anti-HER-2/neu bispecific antibody, MDX-210 (qv), with IDM's macrophage activated killer (MAK) cells, for the potential treatment of ovarian cancer. In May 2002, this 'cell drug' therapy was in phase III trials in a number of European countries, Canada and Australia [ 427441], [ 451031]; these trials were ongoing in May 2003 [ 497916], [ 495455]. However, the therapy was not listed on IDM's pipeline in June 2005 [ 607044].
- Latest Change
- 2006-06-29: 2 references added [676268, 676270]
(Osidem, Osidem-2, MAK + anti-HER2/neu bispecific antibody, MAK + MDX-210)
Update Date: 2006-06-29
http://www.thomson-pharma.com/report/drug?dr=14401&drname=IDM-1
Indication: Ovary tumor
Action: Anticancer; Erbb2 tyrosine kinase receptor inhibitor
Technology: Antibody; Cell therapy
Copyright Thomson Scientific
| Publisher Version | Back to chart | Previous record | Next record |
-
IDM-1
- Development Status
- Development Status - No Development Reported
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Europe Phase III IDM Cancer, ovary North America Phase III IDM Cancer, ovary - Therapeutic Class
- Ovarian Cancer Therapy
- Condition
- Cancer, ovary
- Action
- Anti-HER2/neu/ErbB2; Inhibitors of Signal Transduction Pathways
- Product Milestone History
Product Milestone History Milestone Date Milestone Condition Notes Organization Area Dec, 1999 Phase III Cancer, ovary IDM N. America
(Osidem)
Prous Accession Number: 289382
Originator: IDM
Highest Phase: Phase III
Copyright Prous Science
| Publisher Version | Back to chart | Previous record |
-
MDX-210
- Development Status
- Development Status - No Development Reported
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Europe Phase III Medarex Cancer, ovary Treatment of ovarian cancer United States Phase II Medarex Cancer, breast Treatment of breast cancer United States Phase II Medarex Cancer, colorectal Treatment of colorectal cancer United States Phase II Medarex Cancer, kidney (renal cell carcinoma) Treatment of renal cell carcinoma United States Phase III Medarex Cancer, prostate Treatment of prostate cancer - Therapeutic Class
- Ovarian Cancer Therapy; Colorectal Cancer Therapy; Prostate Cancer Therapy; Breast Cancer Therapy; Renal Cancer Therapy
- Condition
- Cancer, kidney (renal cell carcinoma); Cancer, colorectal; Cancer, prostate; Cancer, breast; Cancer, ovary
- Action
- Anti-HER2/neu/ErbB2; Anti-FCgammaRI (FCGR1; CD64); Inhibitors of Signal Transduction Pathways
(MDX-H210; IDM-1)
Prous Accession Number: 204341
Originator: Medarex
Highest Phase: Phase III
Copyright Prous Science
| Publisher Version | Back to chart | Next record |
-
herstatin
- Investigational Drugs Database 49257
- Companies
- Receptor BioLogix Inc: Originator developing and marketing own product
- Development Status
Company Country Status Indication Date Confidence Receptor BioLogix Inc US Discovery Cancer 2003-07-24
Status: Discovery- Summary
- Receptor BioLogix is investigating herstatin as a potential broad-spectrum anticancer agent. In April 2005, the company planned to start phase I trials in cancers, characterized by high levels of overexpression and activation of the HER family, in mid-2006 [598243].
- Latest Change
- 2005-05-18: Minor editorial amendment
(Dimercept)
CAS Registry No.: 249761-15-7
Update Date: 2005-05-18
http://www.thomson-pharma.com/report/drug?dr=49257&drname=herstatin
Indication: Cancer
Action: Anticancer; Epidermal growth factor receptor modulator; Erbb2 tyrosine kinase receptor inhibitor; Erbb3 tyrosine kinase receptor inhibitor; Erbb4 tyrosine kinase receptor inhibitor; Oncogene inhibitor
Technology: Gene expression regulation; Natural product; Protein (fusion)
Copyright Thomson Scientific
| Back to chart | Previous record | Next record |
-
RB-200h
(Dimercept, Hermodulins, Herstatin)
- Company Status
Company Status Receptor BioLogix Preclinical - Summary
- RB-200h (Hermodulins) is a series of engineered pan-HER ligand Traps, under development by Receptor BioLogix for the treatment of nsclc. They are an Intron Fusion Protein and an alternative product of the HER-2 gene that contains part of the HER-2 protein receptor fused to novel amino-acid sequences encoded by an intron. They are antagonists of erb1, 2 and 3. The series was formerly known as Dimercept and Herstatin. They also have potential in autoimmune diseases (Company Fact Sheet, Receptor BioLogix, 23 Feb 2005; 9th Ann C21 BioVentures (Monterey), 2007).
Marketing
They were licensed exclusively from Oregon Health Sciences University, Portland, OR, the US (Company presentation, Receptor BioLogix, 2005).
Clinical
Phase I
An IND is expected in 2008.
Preclinical
IND candidate selection is expected in Sep 2007. In nude mice xenografts with an HER1, 2 and 3 cell lines, 10 and 30mg/kg resulted in a 44% reduction in tumour growth. In vivo, it was orally-active. In vitro, it demonstrated synergistic activity with tyrosine kinase inhibitors, including erlotinib and gefitinib (both qv). In vitro, it inhibited ligand-induced cell proliferation in cancer cells (9th Ann C21 BioVentures (Monterey), 2007). In nude mice, RB-200h inhibited DU145 prostate cancer at doses comparable to antibody therapeutics. In vivo, it inhibited the growth of SKOV3 ovarian, NCI-N87 gastric and U87 glioblastoma tumours. It was more active cf trastuzumab (qv) against SK-OV3 and NCI-N87 (Company presentation, Receptor BioLogix, 2005).
Licensing
It is available for partnering worldwide (9th Ann C21 BioVentures (Monterey), 2007). Updated by JW on 4/6/2007. - Therapeutic Activity
- Recombinant, other (T2Z)
Anticancer, other (K6Z)
Immunosuppressant (I5) - Pharmacological Activity
- ErbB-1 tyrosine kinase inhibitor (KI-TYE1-AN)
ErbB-2 tyrosine kinase inhibitor (KI-TYE2-AN)
Epidermal growth factor receptor 3 inhibitor (GF-EPR3-AN) - Indications
- Cancer, lung, non-small cell
- Target Names
- epidermal growth factor receptor (erythroblastic leukaemia viral (v-erb-b) oncogene homologue, avian)
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 3 (avian) - Therapy Status
Therapy Pharmacology Status T2Z KI-TYE1-AN KI-TYE2-AN GF-EPR3-AN Preclinical K6Z KI-TYE1-AN KI-TYE2-AN GF-EPR3-AN Preclinical I5 KI-TYE1-AN KI-TYE2-AN GF-EPR3-AN Preclinical - Indication Status
Indication Status Cancer, lung, non-small cell Preclinical - Regional Status
- USA - Preclinical
- Licensing Opportunities
- Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Colombia, Denmark, Finland, France, Germany, Greece, Hong Kong, India, Ireland, Israel, Italy, Japan, Luxembourg, Malaysia, Mexico, Netherlands, New Zealand, Norway, Peru, Philippines, Portugal, Russian Federation, South Africa, South Korea, Spain, Sweden, Switzerland, Thailand, Turkey, UK, USA, Venezuela
- Ratings
- Novelty: 1 - All Preclinical
Market Size: 3 - US$ 2001-5000 million
Development Speed: 1 - Development not started
Total: 0 - Update History
Date Detail 2007-05-24 Development Continuing 2007-05-24 Licensing Opportunities (Worldwide) 2007-05-24 New Indication (Cancer, lung, non-small cell) 2006-11-07 No Development Reported 2005-07-01 Development Continuing 2005-02-04 No Development Reported 2003-10-06 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 36857
Last Update: 2007-06-04
Originator: Receptor BioLogix (USA)
World Status: Preclinical
Origin of Material: Biological, protein, recombinant (BI-P-R)
Route of Administration: Unknown (UN)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record |
-
DIMERCEPT
- IMS R&D Focus Accession Number: 2021354
- Latest Information
- 18 February 2008: Receptor Biologix is no longer developing DIMERCEPT for the treatment of cancer. Preclinical studies were under way in the USA.
- Commercial Summary
- Receptor BioLogix was developing DIMERCEPT, an epidermal growth factor receptor (EGFR) and HER2 antagonist, for the potential treatment of cancer; however, development is no longer ongoing (Receptor BioLogix, FEB 2008). DIMERCEPT, an intron fusion protein, is composed of coding sequences from the normal HER2 protein fused to amino acid sequences encoded by intron sequences (Receptor BioLogix, OCT 2003). The compound was undergoing preclinical evaluation in the USA (Receptor BioLogix, OCT 2003). The company anticipated phase I trials of the agent in the treatment of gastric cancer to begin during 2005 (Receptor BioLogix, OCT 2003). DIMERCEPT had been licensed exclusively to Receptor BioLogix from Oregon Health and Science University (USA). Receptor BioLogix was seeking partners for further development of the agent (Receptor BioLogix, OCT 2003).
- Scientific Summary
- In vitro, DIMERCEPT showed antagonist activity against tumor cells overexpressing either or both the epidermal growth factor receptor (EGFR) or HER2, and did not affect normal cells or tumor cells that do not express HER receptors. DIMERCEPT also showed superior activity to trastuzumab against ovarian and gastric cancer cells (Receptor Bio Logix, OCT 2003). In vivo, DIMERCEPT showed efficacy in animal models of ovarian and prostate cancer with no adverse effects, such as weight loss, being observed. In a nude mouse model of hormone-resistant prostate cancer, the compound was administered three times a week at either 10 mg/kg or 30 mg/kg. Tumors were eradicated in mice treated with 30 mg/kg DIMERCEPT (Receptor BioLogix, OCT 2003).
- Indications
- cancer
solid tumor - Therapeutic Class
- All Other Antineoplastics (L1X9)
- Mechanism of Action
- biotechnology
fusion protein
EGF receptor inhibitor
protein
recombinant protein - Indication Status
Country Indication Phase USA solid tumor Discontinued - Franchise
Company Parent Relation Region Oregon Health Sciences University Oregon Health Sciences University licensor Receptor BioLogix Receptor BioLogix licensor - Update History
Date Detail DATE UNKNOWN Discontinued. OCT 2003 Preclinical, USA (solid tumors). Available for licensingworldwide.
Publication Date: 2008-02-15
Originator: Oregon Health Sciences University (USA); Receptor BioLogix (USA)
Highest Phase: Discontinued
Copyright IMS World Publications
| Publisher Version | Back to chart | Next record |
-
Anti-HER2 antibody
(Zemab)
- Drug Development Phase
Indication Phase Country Cancer Phase I Denmark - Properties
- Mechanism Of Action: HER2 inhibitors
Route of Administration: Intratumoural - Commercial Introduction
- TopoTarget is developing, under a worldwide license from Novartis, an antibody-toxin targeting the HER2 receptor for the treatment of cancer, specifically breast cancer, but also cancer of the head and neck, in which the HER2 receptor is also thought to play a role. The antibody candidate has shown potential in preclinical models and following direct injection into HER2-positive tumours, reduced tumour size in 6 out of 10 patients in a phase I trial. The product, also known as Zemab?, is undergoing GMP manufacturing in preparation for the initiation of additional clinical trials during the fourth quarter of 2007.
- Drug Development History
Date Event 15 May 2007 New profile 15 May 2007 Phase-I clinical trials in Cancer in Denmark (Intratumoural)
Last Update: 2007-07-06
Adis R&D Insight accesion number: 800026315
Indications: Cancer
Therapeutic Class (WHO): Antineoplastic Agents (L01)
Therapeutic Class (EphMRA): Antineoplastics (L1)
Originator: Novartis
Licensee: TopoTarget
Highest Phase: Phase I
© 2008 Adis Data Information BV
| Publisher Version | Back to chart | Previous record | Next record |
-
Zemab
- Development Status
- Development Status - Launched, Registered or Under Active Development
Country/Area Phase Organization Brand Name Condition Indication Admin. Route Formulation Phase I TopoTarget Cancer, head and neck - Therapeutic Class
- Head and Neck Cancer Therapy; Oncolytic Drugs
- Condition
- Cancer, head and neck; Cancer
- Action
- Anti-HER2/neu/ErbB2
- Summary
- Zemab(R) is a recombinant protein targeting ErbB2/HER2 that is currently in early clinical trials at TopoTarget for the treatment of cancer. In 2003, Novartis offered TopoTarget an option to exclusively in-license Zemab(R) for further development, an option which TopoTarget subsequently exercised.
Prous Accession Number: 393112
Licensee: TopoTarget; Novartis
Highest Phase: Phase I
Copyright Prous Science
| Back to chart | Previous record | Next record |
-
Zemab
(anticancer antibody, G2M)
- Company Status
Company Status Novartis Phase II Clinical Trial TopoTarget Phase II Clinical Trial - Summary
- Zemab is an antibody derivative which targets HER2-positive cancer cells, under development by TopoTarget (G2M Cancer Drugs before the acquisition) for the treatment of breast and head and neck cancers. It contains an antibody-derived recognition domain and an additional cell-killing domain (Company Web Page, TopoTarget, 26 Apr 2005).
Marketing
TopoTarget exclusively in-licensed Zemab from Novartis in 2003, and executed an in-licensing option in Mar 2007 for exclusive development and marketing rights. Novartis is to receive milestone payments and royalities (3rd Qtr Rep, TopoTarget, 2006; Direct communication TopoTarget, 17 Nov 2006; Press release, TopoTarget, 12 Mar 2007).
Clinical
Phase II
It is in Phase II trials for breast and head and neck cancer (BIO 2006 (Chicago)). Further trials are planned for the 4th qtr of 2007 (Ann Rep, TopoTarget, 2006).
Phase I
In a Phase I trial, Zemab iv injected directly into a tumour demonstrated a reduction in tumour size in 6/10 patients. Further trials are planned for 2007 (Press release, TopoTarget, 1 Mar 2005; 3rd Qtr Rep, TopoTarget, 2006).
Preclinical
It is covered by patents in all major territories (Ann Rep, TopoTarget, 2006).
Licensing
It is available for licensing worldwide (2nd BIO-Europe Spring (Madrid), 2008). Updated by JS on 14/4/2008. - Therapeutic Activity
- Immunotoxin (T3B1)
Anticancer, immunological (K3) - Pharmacological Activity
- Epidermal growth factor receptor 2 antagonist (GF-EPR2-AN)
- Indications
- Cancer, breast
Cancer, head and neck - Target Names
- v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2, neuro/glioblastoma derived oncogene homologue (avian)
- Therapy Status
Therapy Pharmacology Status T3B1 GF-EPR2-AN Phase II Clinical Trial K3 GF-EPR2-AN Phase II Clinical Trial - Indication Status
Indication Status Cancer, breast Phase II Clinical Trial Cancer, head and neck Phase II Clinical Trial - Regional Status
- Denmark - Phase II Clinical Trial
- Licensing Opportunities
- Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, China, Colombia, Denmark, Finland, France, Germany, Greece, Hong Kong, India, Ireland, Israel, Italy, Japan, Luxembourg, Malaysia, Mexico, Netherlands, New Zealand, Norway, Peru, Philippines, Portugal, Russian Federation, South Africa, South Korea, Spain, Sweden, Switzerland, Thailand, Turkey, UK, USA, Venezuela
- Ratings
- Novelty: 6 - Leading Compound
Market Size: 3 - US$ 2001-5000 million
Development Speed: 4 - Faster than Average
Total: 13 - Update History
Date Detail 2008-04-14 Licensing Opportunities (Worldwide) 2006-04-24 Change in Status (Phase II Clinical Trial) 2005-04-26 Pharmacology Identified (ErbB-2 inhibitors (KI-TYE2-)) 2005-04-26 Target Identified (v-erb-b2 (2064)) 2005-03-02 New Product in Pharmaprojects
PJB Pharmaprojects Accession Number: 39921
Last Update: 2008-04-14
Originator: Novartis (Switzerland)
Licensee: TopoTarget (Denmark)
World Status: Phase II Clinical Trial
Origin of Material: Biological, protein, recombinant (BI-P-R)
Route of Administration: Parenteral, intratumoural (P-TU)
Copyright PJB Publications, Richmond, Surrey, UK
| Back to chart | Previous record |
-
MAb, ErbB2/HER2, TopoTarget
(ZEMAB)
- IMS R&D Focus Accession Number: 2024242
- Patent Summary
- Patent search conducted - no relevant patent identified (OCTOBER 2005).
- Latest Information
- 19 March 2007: On 12 March 2007 TopoTarget announced that it has exercised its licensing option with Novartis, to acquire exclusive worldwide rights for the development of ZEMAB. Under the original agreement between G2M Cancer Drugs (acquired by TopoTarget in March 2005) and Novartis, signed in January 2003, TopoTarget was granted the option to acquire an exclusive license to patent rights, and an interest in joint patent rights relating to ZEMAB. ....ZEMAB, a recombinant product comprising anti-HER2 antibody-derived specific recognition domain combined with pseudomonal exotoxin, is being developed for the treatment of breast cancer, and head and neck cancer. Phase I trials evaluating intratumoral and iv dosing of ZEMAB have been conducted. TopoTarget plans to conduct further clinical trials.
- Commercial Summary
- TopoTarget is developing ZEMAB, a recombinant antibody-based compound directed against ErbB2/HER2, a common neck cancer antigen, for the potential treatment of breast cancer, and head and neck cancer. ZEMAB is an immunotoxin comprising an antiHER2 antibody-derived specific recognition domain combined with pseudomonal exotoxin. Phase I trials to evaluate systemic and intratumoral dosing of ZEMAB have been conducted. In January 2003, G2M Cancer Drugs (now known as TopoTarget) signed an agreement with Novartis for the development of ZEMAB, and was granted the option to acquire an exclusive license to patent rights, and an interest in joint patent rights relating to ZEMAB. Topo Target acquired certain rights to ZEMAB with the acquisition of its original developer, G2M Cancer Drugs AG, in March 2005. In March 2007, TopoTarget exercised the option with Novartis for exclusive worldwide development rights to ZEMAB.
R&D progress
Phase I trials to evaluate systemic dosing of ZEMAB have completed and the maximum tolerated dose has been established (TopoTarget, MAR 2005) . Compassionate use trials have been conducted to evaluate intratumoral injections of ZEMAB in patients with breast cancer, and head and neck cancer, and results have been reported (TopoTarget, MAY 2005). Phase II trials of ZEMAB in patients with breast cancer, and head and neck cancer are planned (TopoTarget, FEB 2006). TopoTarget confirmed phase I, cancer, SEP 2005.
Licensing/Partnering
In January 2003, G2M Cancer Drugs (now known as TopoTarget) signed an agreement with Novartis, for the development of a recombinant protein targeting ErbB2/HER2. Under the agreement, Novartis granted G2M Cancer Drugs an option to acquire an exclusive license to patent rights, and an interest in joint patent rights relating to ZEMAB. G2M Cancer Drugs is to develop the product during the option period, and will make an upfront payment, in addition to milestone and royalty payments on development of the product, if the option is exercised. Upon completion of a phase II trial, Novartis has the option to re-acquire rights and continue development of ZEMAB (TopoTarget, MAY 2005). Topo Target has exercised its option agreement with Novartis, under which it has acquired worldwide exclusive rights for the development of ZEMAB (TopoTarget, MAR 2007). TopoTarget acquired certain rights to ZEMAB with the acquisition of its original developer, G2M Cancer Drugs AG, in March 2005 (Topo Target, MAR 2005).
Availability
TopoTarget is seeking partners for ZEMAB, worldwide excluding Europe (TopoTarget, FEB 2006).
Clinical data
In a compassionate use trial, intratumoral injection of ZEMAB in patients with breast cancer, and head and neck cancer resulted in a reduction in tumor size in six out of ten patients (TopoTarget, MAY 2005). - Indications
- cancer
solid tumor
breast cancer
head and neck cancer - Therapeutic Class
- Antineoplastic Monoclonal Antibodies (L1X3)
- Mechanism of Action
- biotechnology
monoclonal antibody
monoclonal antibody fragment
immunotoxin
fusion toxin - Indication Status
Country Indication Phase Denmark cancer Phase I Denmark solid tumor Phase I Denmark breast cancer Phase I Denmark head and neck cancer Phase I - Licensing Opportunities
- Available for licensing in: Worldwide. Unavailable for licensing in: Europe.
- Licensing Contact
- Nicholas la Thangue,
Chief Business Development Officer,
TopoTarget,
87A Milton Park,
Abingdon,
Oxon,
OX14 4RY,
UK;
Tel: +44 1235 443 700;
Fax: +44 1235 835 557;
Email: n.lathangue@topotarget.co.uk. - Franchise
Company Parent Relation Region Novartis Novartis licensor TopoTarget TopoTarget licensee - Update History
Date Detail MAR 2007 TopoTarget exercises option under agreement with Novartisfor exclusive worldwide development rights. FEB 2006 Available for partnering, Worldwide excluding Europe. MAR 2005 Phase I, Denmark. TopoTarget acquires G2M Cancer Drugs. JAN 2003 Agreement between Novartis and G2M Cancer Drugs.
Publication Date: 2007-01-01
Originator: Novartis (Switzerland)
Licensee: TopoTarget (Denmark)
Highest Phase: Phase I
Copyright IMS World Publications